To investigate the association between admission blood glucose levels and risk of in-hospital cardiovascular and renal complications.
In this multicenter prospective study of 36,269 adults hospitalized with COVID-19 between 6 February 2020 and 16 March 2021 (N = 143,266), logistic regression models were used to explore associations between admission glucose level (mmol/L and mg/dL) and odds of in-hospital complications, including heart failure, arrhythmia, cardiac ischemia, cardiac arrest, coagulation complications, stroke, and renal injury. Nonlinearity was investigated using restricted cubic splines. Interaction models explored whether associations between glucose levels and complications were modified by clinically relevant factors.
Cardiovascular and renal complications occurred in 10,421 (28.7%) patients; median admission glucose level was 6.7 mmol/L (interquartile range 5.8–8.7) (120.6 mg/dL [104.4–156.6]). While accounting for confounders, for all complications except cardiac ischemia and stroke, there was a nonlinear association between glucose and cardiovascular and renal complications. For example, odds of heart failure, arrhythmia, coagulation complications, and renal injury decreased to a nadir at 6.4 mmol/L (115 mg/dL), 4.9 mmol/L (88.2 mg/dL), 4.7 mmol/L (84.6 mg/dL), and 5.8 mmol/L (104.4 mg/dL), respectively, and increased thereafter until 26.0 mmol/L (468 mg/dL), 50.0 mmol/L (900 mg/dL), 8.5 mmol/L (153 mg/dL), and 32.4 mmol/L (583.2 mg/dL). Compared with 5 mmol/L (90 mg/dL), odds ratios at these glucose levels were 1.28 (95% CI 0.96, 1.69) for heart failure, 2.23 (1.03, 4.81) for arrhythmia, 1.59 (1.36, 1.86) for coagulation complications, and 2.42 (2.01, 2.92) for renal injury. For most complications, a modifying effect of age was observed, with higher odds of complications at higher glucose levels for patients age <69 years. Preexisting diabetes status had a similar modifying effect on odds of complications, but evidence was strongest for renal injury, cardiac ischemia, and any cardiovascular/renal complication.
Increased odds of cardiovascular or renal complications were observed for admission glucose levels indicative of both hypo- and hyperglycemia. Admission glucose could be used as a marker for risk stratification of high-risk patients. Further research should evaluate interventions to optimize admission glucose on improving COVID-19 outcomes.
Introduction
As of December 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected >267 million people and claimed >5.2 million lives worldwide. After infection, the course of the disease (coronavirus disease 2019 [COVID-19]) varies, ranging from asymptomatic mild infection to severe complications and death. People who require hospital admission have the worse outcomes, with a mortality risk of 10–26% in the U.S. and U.K. (1,2).
Individuals with certain chronic comorbidities, e.g., obesity, hypertension, cardiovascular disease, and diabetes, have increased susceptibility to severe COVID-19 (3,4), with >25% of patients hospitalized with COVID-19 having at least one of these comorbidities. Diabetes in particular has been shown to increase the risk of adverse outcomes in individuals with COVID-19 (4–6). A study of >61 million people in England reported that both type 1 and type 2 diabetes are associated with a more than three- and twofold increase in the odds of COVID-19–related in-hospital mortality, respectively (7). Another study also showed that preadmission HbA1c is associated with an increased risk of in-hospital COVID-19–related mortality (8), while a positive relationship between plasma glucose on admission and in-hospital COVID-19 mortality has also been observed (9). These findings of heightened risks of adverse outcomes in individuals with high glucose levels are expected given the hyperglycemia-induced disruptions to the immune system that have been observed (10). Conversely, overly intensive glycemic control may lead to severe hypoglycemia, which has also been associated with an increased risk of mortality in non–COVID-19 illness (11,12). As such, it is vital to understand how the risk for adverse COVID-19–related outcomes changes across the glucose spectrum.
In addition to the baseline risk associated with cardiometabolic conditions such as diabetes, recent studies have shown that COVID-19 can cause acute cardiovascular injury, including arrhythmias, cardiac arrest, myocardial infarction, and heart failure (13,14). These insults can in turn lead to chronic cardiovascular damage or death, even in patients without existing cardiovascular disease (15,16). The mechanisms linking COVID-19 with cardiovascular complications are many and include the release of cytokines (cytokine storm), dysregulation of the renin-angiotensin-aldosterone and coagulation systems, and plaque rupture during the acute infection phase (17). However, it has been suggested that the diabetes-related changes to the immune system and renin-angiotensin-aldosterone system, namely an imbalanced expression of ACE2 paired with inflammation, oxidative stress, and endothelial dysfunction, may contribute to an increased vascular permeability and/or cytokine storm (18,19), thus providing a mechanism underpinning the increased risk of cardiovascular dysfunction observed in these patient groups.
Because people with hyperglycemia often have a combination of high-risk characteristics, e.g., older age, obesity, and ethnic minority status, it is important to understand how these characteristics interact to modify the risk of COVID-19 complications in people with hyperglycemia. To unpick the relative importance of the various factors contributing to adverse COVID-19 prognosis, large, well-phenotyped samples are required, with good coverage of these contributing factors. Accordingly, this study investigated the association between glucose levels on admission and the risk of in-hospital cardiovascular and renal complications in a large, nationally representative sample of people hospitalized for COVID-19. Furthermore, we investigated whether the associations between glucose levels and complications were different by age, sex, ethnicity, obesity, and preexisting diabetes diagnoses.
Research Design and Methods
Population
We used data from the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) World Health Organization (WHO) Clinical Characterization Protocol UK (CCP-UK) for Severe Emerging Infection. Developed by ISARIC and WHO in 2009, the protocol was reactivated on 17 January 2020 in response to the SARS-CoV-2 pandemic. The protocol and all study materials for this actively recruiting prospective cohort can be accessed online (https://isaric4c.net). The study was approved by the South Central–Oxford C Research Ethics Committee in England (Ref. no. 13/SC/0149) and by the Scotland Research Ethics Committee (Ref. no. 20/SS/0028). The ISARIC WHO CCP-UK study was registered at https://www.isrctn.com/ISRCTN66726260 and designated as an urgent public health research study by the National Institute for Health Research.
Sample
Patients aged ≥18 years who were admitted to the hospital between 6 February 2020 and 16 March 2021 with a confirmed COVID-19 diagnosis were included. Patients were excluded if they had missing outcome or sex data or were recipients of an organ transplant or immunosuppression therapies or had rare diseases likely to significantly increase the risk of infections (e.g., severe combined immunodeficiency). As of 16 March 2021, 143,266 patients were included in the ISARIC WHO CCP-UK study, of whom 140,685 (98.2%) had a confirmed diagnosis of COVID-19. Of these, 35,601 (25.3%) had the relevant outcome, exposure, and covariate data for analysis (excluded n = 107,665; 75.2%). Supplementary Fig. 1 shows how the final sample was derived. Differences in key clinical characteristics between those included and excluded are provided in Supplementary Table 1.
Data were directly transcribed from routine health care into case report forms hosted on a REDCap database (Research Electronic Data Capture, https://projectredcap.org). Data collection was undertaken by research nurses, administrators, and medical students. Detailed demographic and clinical data were collected on admission, with follow-up data on clinical care collected at days 3, 6, and 9 and discharge or status at 28 days if not discharged.
Exposures
Blood glucose levels were assessed via a random venous glucose sample collected from patients on hospital admission by a health care professional. Blood samples were analyzed in National Health Service (NHS) clinical laboratories, and usual processing standard operating procedures were followed. Blood glucose was available as a continuous variable and measured in mmol/L. For descriptive purposes, patients were classified as having hypoglycemia (≤3.9 mmol/L [70.2 mg/dL]), normoglycemia (4–11 mmol/L [72–198 mg/dL]), or hyperglycemia (≥11.1 mmol/L [199.8 mg/dL]) on the basis of their random admission glucose (20).
Covariates
Sex, as recorded at birth, was coded as female or male. Age was measured to the nearest year using the difference between date of birth and the admission date. For this analysis and because of low numbers, ethnicity was coded into four distinct groups, reflecting the most prevalent ethnic groups in the 2011 census in England and Wales (21), as follows: White, South Asian, Black, and other (East and West Asian, Arab, Latin American, Aboriginal/First Nations, other). Obesity was coded as yes or no on the basis of clinical assessment from the attending clinician. This assessment was based on an objective measurement of obesity, e.g., BMI ≥30 kg/m2 or abdominal girth, or on subjective clinical judgment. Preexisting diabetes status (yes or no) was based on an existing clinician diagnosis of the condition in patient records.
Outcomes
In-hospital cardiovascular complications were extracted from routine clinical records by local investigators and included stroke, heart failure, arrhythmia, cardiac ischemia, cardiac arrest, and coagulation disorders (abnormal coagulation identified by abnormal prothrombin time or activated partial thromboplastin time). Because of the intrinsic nature of cardiovascular and kidney dysfunction, we also included acute renal injury in the list of complications. A dichotomous variable representing any cardiovascular/renal complication was derived to identify patients with at least one of the in-hospital complications listed. Further information regarding how these variables were defined can be found in the Supplementary Material.
Statistical Analysis
Descriptive statistics are presented as number (%) and median (25th and 75th centiles) for categorical and continuous data, respectively. We used logistic regression models to investigate whether the odds of in-hospital complications and mortality differed across admission glucose levels. Nonlinear associations were investigated using restricted cubic splines, which were developed specific to each outcome and unadjusted for covariates (model 1). We tested spline models with 2, 3, 4, and 5 knots located at equally spaced centiles across the glucose distribution. The Bayesian information criterion was used for selection between models with a different number of knots, with a lower value indicative of improved fit. To aid interpretation, splines were centered on a reference value of 5 mmol/L. In subsequent models, we progressively adjusted for key demographic variables, including age (entered as a restricted cubic spline), sex, and ethnicity (model 2); obesity status (model 3); and diabetes status (model 4). In a final model, we adjusted for oxygen saturation at admission as a proxy for COVID-19 severity (model 5).
Using model 5, we then explored interactions to identify whether the association between admission glucose and each outcome was modified by clinically relevant factors, namely sex, ethnicity, age, obesity, and preexisting diabetes status. To aid interpretation, interactions with age were performed with a dichotomized age variable (based on the mean of the sample of 69 years). Interactions were formally tested using the likelihood ratio test for nested models, comparing models with and without the interaction term; P < 0.05 indicated whether the interaction terms should be retained.
As supplementary analyses, we investigated the associations using the categorical hypoglycemia, normoglycemia, and hyperglycemia variable in logistic regression models, with patients categorized as having normoglycemia as the reference. We also repeated the analyses using a variable representing any cardiovascular/renal complication or all-cause death to account for the competing risk of death in assessing in-hospital complications.
All analyses were conducted using Stata 16 software (StataCorp, College Station, TX). The statistical code for the analyses in this article is publicly available at GitHub (repository URL: https://github.com/tomnorris1988/Diabetes-Care.git).
Results
Descriptive statistics of the sample (n = 35,601) are shown in Table 1. The sample comprised 20,591 males (56.8%), had a median age of 71 years (interquartile range [IQR] 57–82), and comprised 29,580 individuals (81.6%) of White ethnicity (Table 1). Median glucose level on admission was 6.7 mmol/L (IQR 5.8–8.7) (120.6 mg/dL [104.4–156.6]). More than one-quarter of the sample experienced a cardiovascular or renal complication while hospitalized (n = 10,421; 28.7%) of whom 5729 (55.0%) survived and 4691 (45.0%) died. Characteristics of the sample of patients included versus excluded from the analysis are shown in Supplementary Table 1. Imbalances between the samples were observed, with the included sample being younger (68.6 vs. 70.8 years), more likely to be from a Black or minority ethnic group (18.4% vs. 14.4%), and having a preexisting diabetes diagnosis (25.4% vs. 19.1%). Patient characteristics of those who, at 28 days after admission, had been discharged versus remained hospitalized or were transferred to another facility are provided in Supplementary Table 2. Imbalances were observed in age and number of cardiovascular/renal complications, with the group remaining hospitalized or transferred to another facility being older (70.0 vs. 65.0 years) and experiencing a higher proportion of cardiovascular/renal complications (41.0% vs. 19.5%) than those who had been discharged within 28 days.
Patient characteristics stratified by presence of cardiovascular/renal complications
| . | Total sample (n = 36,269) . | Patients not experiencing a cardiovascular/renal complication (n = 25,848) . | Patients experiencing a cardiovascular/renal complication (n = 10,421) . |
|---|---|---|---|
| Sex | |||
| Male | 20,591 (56.8) | 14,147 (54.7) | 6,444 (61.8) |
| Female | 15,678 (43.2) | 11,701 (45.3) | 3,977 (38.2) |
| Age on admission (years) | 71 (57–82) | 69 (55–81) | 75 (63–84) |
| Ethnicity | |||
| White | 29,580 (81.6) | 21,069 (81.5) | 8,511 (81.7) |
| South Asian | 2,637 (7.3) | 1,917 (7.4) | 720 (6.9) |
| Black | 1,285 (3.5) | 833 (3.2) | 452 (4.3) |
| Other | 2,767 (7.6) | 2,029 (7.9) | 738 (7.1) |
| Glucose on admission | |||
| mmol/L | 6.7 (5.8–8.7) | 6.6 (5.7–8.3) | 7.2 (6.0–9.5) |
| mg/dL | 120.6 (104.4–156.6) | 118.8 (102.6–149.4) | 129.6 (108.0–171.0) |
| Obesity | 5,680 (15.7) | 3,710 (14.4) | 1,970 (18.9) |
| Preexisting diabetes | 9,202 (25.37) | 5,933 (23.0) | 3,269 (31.4) |
| In-hospital cardiovascular/renal complications | |||
| Arrhythmia | 2,967 (28.5) | — | 2,967 (28.5) |
| Cardiac ischemia | 551 (5.3) | — | 551 (5.3) |
| Cardiac arrest | 920 (8.8) | — | 920 (8.8) |
| Coagulation complications | 1,625 (15.6) | — | 1,625 (15.6) |
| Stroke | 470 (4.5) | — | 470 (4.5) |
| Heart failure | 1,282 (12.3) | — | 1,282 (12.3) |
| Renal injury | 6,458 (62.0) | — | 6,458 (62.0) |
| Outcome status at 28 days postadmission | |||
| Discharged alive | 23,662 (65.2) | 19,123 (74.0) | 4,539 (43.6) |
| Remained hospitalized | 859 (2.4) | 424 (1.6) | 435 (4.2) |
| Transferred to another facility | 1,632 (4.5) | 1,046 (4.1) | 586 (5.6) |
| Palliative discharge | 490 (1.4) | 321 (1.2) | 169 (1.6) |
| Died | 9,618 (26.5) | 4,927 (19.1) | 4,691 (45.0) |
| . | Total sample (n = 36,269) . | Patients not experiencing a cardiovascular/renal complication (n = 25,848) . | Patients experiencing a cardiovascular/renal complication (n = 10,421) . |
|---|---|---|---|
| Sex | |||
| Male | 20,591 (56.8) | 14,147 (54.7) | 6,444 (61.8) |
| Female | 15,678 (43.2) | 11,701 (45.3) | 3,977 (38.2) |
| Age on admission (years) | 71 (57–82) | 69 (55–81) | 75 (63–84) |
| Ethnicity | |||
| White | 29,580 (81.6) | 21,069 (81.5) | 8,511 (81.7) |
| South Asian | 2,637 (7.3) | 1,917 (7.4) | 720 (6.9) |
| Black | 1,285 (3.5) | 833 (3.2) | 452 (4.3) |
| Other | 2,767 (7.6) | 2,029 (7.9) | 738 (7.1) |
| Glucose on admission | |||
| mmol/L | 6.7 (5.8–8.7) | 6.6 (5.7–8.3) | 7.2 (6.0–9.5) |
| mg/dL | 120.6 (104.4–156.6) | 118.8 (102.6–149.4) | 129.6 (108.0–171.0) |
| Obesity | 5,680 (15.7) | 3,710 (14.4) | 1,970 (18.9) |
| Preexisting diabetes | 9,202 (25.37) | 5,933 (23.0) | 3,269 (31.4) |
| In-hospital cardiovascular/renal complications | |||
| Arrhythmia | 2,967 (28.5) | — | 2,967 (28.5) |
| Cardiac ischemia | 551 (5.3) | — | 551 (5.3) |
| Cardiac arrest | 920 (8.8) | — | 920 (8.8) |
| Coagulation complications | 1,625 (15.6) | — | 1,625 (15.6) |
| Stroke | 470 (4.5) | — | 470 (4.5) |
| Heart failure | 1,282 (12.3) | — | 1,282 (12.3) |
| Renal injury | 6,458 (62.0) | — | 6,458 (62.0) |
| Outcome status at 28 days postadmission | |||
| Discharged alive | 23,662 (65.2) | 19,123 (74.0) | 4,539 (43.6) |
| Remained hospitalized | 859 (2.4) | 424 (1.6) | 435 (4.2) |
| Transferred to another facility | 1,632 (4.5) | 1,046 (4.1) | 586 (5.6) |
| Palliative discharge | 490 (1.4) | 321 (1.2) | 169 (1.6) |
| Died | 9,618 (26.5) | 4,927 (19.1) | 4,691 (45.0) |
Data are n (%) or median (IQR).
Admission Glucose and Cardiovascular and Renal Complications
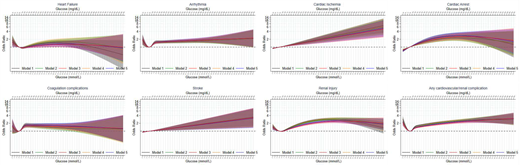
For all complications except cardiac ischemia and stroke, there was a nonlinear association between admission glucose and cardiovascular and renal complications (Fig. 1). For heart failure, arrhythmia, coagulation complications, and renal injury, the pattern of association with glucose, adjusted for all covariates (i.e., model 5), was such that odds of experiencing the outcome decreased to a nadir at 6.4 mmol/L (115 mg/dL), 4.9 mmol/L (88.2 mg/dL), 4.7 mmol/L (84.6 mg/dL), and 5.8 mmol/L (104.4 mg/dL), respectively, and increased thereafter. For heart failure, coagulation complications, and renal injury, odds increased until 26.0 mmol/L (468 mg/dL), 8.5 mmol/L (153 mg/dL), and 32.4 mmol/L (583.2 mg/dL), respectively, with a subsequent plateau, while odds of arrhythmia continued to increase until 50 mmol/L (900 mg/dL). Compared with 5 mmol/L (90 mg/dL), the odds ratios at these glucose levels were 1.28 (95% CI 0.96, 1.69) for heart failure, 2.23 (1.03, 4.81) for arrhythmia, 1.59 (1.36, 1.86) for coagulation complications, and 2.42 (2.01, 2.92) for renal injury. The association between glucose and the odds of experiencing cardiac arrest, cardiac ischemia, and stroke was characterized by increasing odds of the outcome with increasing admission glucose. For cardiac ischemia and stroke, the increase in odds was linear across the glucose distribution, whereas for cardiac arrest, the rate of increase diminished at higher glucose values. Accordingly, the odds of experiencing cardiac arrest, cardiac ischemia, and stroke at the 95th centile of admission glucose levels (i.e., 16.5 mmol/L [297 mg/dL]) were 2.06 (95% CI 1.63, 2.59), 1.51 (1.25, 1.82), and 1.35 (1.09, 1.68) times higher, respectively, compared with 5 mmol/L.
Associations between admission glucose level (in mmol/L and mg/dL) at admission and odds of in-hospital cardiovascular and renal complications.
Associations between admission glucose level (in mmol/L and mg/dL) at admission and odds of in-hospital cardiovascular and renal complications.
Effect Modification by Ethnicity, Age, Sex, Obesity, and Diabetes Status
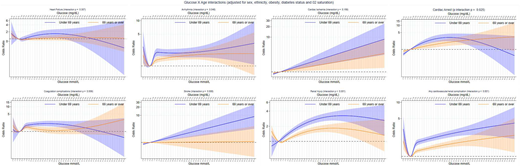
Evidence for a modifying effect of age was observed consistently across all complications except heart failure and cardiac ischemia (Fig. 2). In general, this manifested in a greater effect size for the association between higher admission glucose levels and complications in those <69 years of age; however, at the very highest glucose levels, this association reversed for heart failure, cardiac arrest, and coagulation complications, although CIs at these extreme values were wide and overlapping. At lower glucose levels (<5 mmol/L [90 mg/dL]), the pattern of effect modification across the complications was less consistent. There was also a consistent pattern of effect modification by diabetes status, although the strength of evidence varied across the complications. In all but the very high glucose values (i.e., >97.5th centile, 20.1 mmol/L [361.6 mg/dL]), this pattern of modification was broadly characterized as a greater effect size for the association between higher admission glucose levels and complications in patients without a previous diagnosis of diabetes (Fig. 3). Evidence was strongest for renal injury, cardiac ischemia, and the any cardiovascular/renal complication variable. At the very highest glucose levels (i.e., >97.5th centile) and for heart failure, cardiac arrest, coagulation complications, renal injury, and the any cardiovascular/renal complication variable, however, the pattern of association between admission glucose and diabetes status reversed. The pattern of effect modification across the complications was less consistent at lower glucose levels (e.g., <5 mmol/L [90 mg/dL]). Evidence for a modifying effect of ethnicity was only found for heart failure and cardiac arrest, although a consistent pattern of modification was not observed across these complications (Supplementary Fig. 2). We observed no evidence of a modifying effect of sex (Supplementary Fig. 3) or obesity status (Supplementary Fig. 4) on the relationship between admission glucose levels and any of the complications.
Associations between admission glucose level (mmol/L and mg/dL) and odds of in-hospital cardiovascular and renal complications in patients age <69 years vs. those ≥69 years.
Associations between admission glucose level (mmol/L and mg/dL) and odds of in-hospital cardiovascular and renal complications in patients age <69 years vs. those ≥69 years.
Associations between admission glucose level (mmol/L and mg/dL) and odds of in-hospital cardiovascular and renal complications by preexisting diabetes status.
Associations between admission glucose level (mmol/L and mg/dL) and odds of in-hospital cardiovascular and renal complications by preexisting diabetes status.
Supplementary Analyses
Characteristics of patients stratified by glycemia status are provided in Supplementary Table 3, and the results of the analyses comparing the odds of each outcome in patients classified as having hypo- and hyperglycemia (vs. normoglycemia) are shown in Supplementary Fig. 5. Increased odds of experiencing the complications were observed in patients classified as having hyperglycemia, and this finding was consistent across all the complications. In addition, while point estimates for five of the eight complications (any cardiovascular/renal complication, cardiac arrest, heart failure, cardiac ischemia, and renal injury) suggested an increased likelihood of the outcome in those with hypoglycemia (vs. normoglycemia), the small numbers in this group resulted in imprecise CIs.
We observed a consistent pattern of results with the main findings when rerunning the analyses on a composite outcome representing any cardiovascular/renal complication or all-cause death, i.e., a nonlinear association with admission glucose levels characterized by an increased odds of the outcome at both high and low admission glucose levels (Supplementary Fig. 6). In addition and as per the main analysis, there was evidence that this association was modified by age and diabetes status (Supplementary Fig. 7). Furthermore, increased odds of this outcome were observed in patients classified as having either hypo- or hyperglycemia (Supplementary Fig. 8).
Conclusions
Summary of Findings
Using one of the largest in-hospital samples of patients with COVID-19 to date, we investigated the relationship between glucose levels on hospital admission and a diverse range of in-hospital cardiovascular and renal complications and death. In 35,601 hospitalized patients with COVID-19, 29% experienced an in-hospital cardiovascular or renal complication. For most complications, we observed a nonlinear relationship between admission glucose and the likelihood of experiencing the outcome. However, the nature of the nonlinear relationship was outcome specific, with the greatest likelihood of experiencing heart failure, arrhythmia, and coagulation complications associated with glucose levels in the hypoglycemic range, while for the other complications, the greatest likelihood was observed at much higher glucose levels. Furthermore, for stroke and cardiac ischemia, a linear relationship between the odds of experiencing either of these complications and admission glucose was observed, with increasing odds for higher glucose levels. We also found that a number of these associations may be modified by age and diabetes status, with stronger effects observed in those of younger age and without a preexisting diagnosis of diabetes.
Interpretation of Findings
Blood glucose levels and a previous diagnosis of diabetes are now established risk factors for COVID-19 severity and mortality (7,8,22–25). However, there is much less evidence regarding the associations between admission blood glucose levels and complications indicative of severe COVID-19 that may ultimately result in death. We are aware of only three studies that have investigated associations between admission blood glucose levels and cardiovascular and renal complications (e.g., cardiac and renal injury, coagulation complications) (26–28). In a study of 7,337 patients with COVID-19 in China, Zhu et al. (26) observed that patients with well-controlled blood glucose (70.2–180 mg/dL) experienced less frequent occurrences of acute heart (1.4% vs. 9.9%) and kidney injury (0.7% vs. 3.8%) and disseminated intravascular coagulation (0.0% vs. 0.6%) than patients with poorly controlled blood glucose. Conversely, in a study of 1,544 patients with COVID-19 in the U.S., Klonoff et al. (28) observed that the greatest risk of kidney injury occurred in patients with hypoglycemia (<3.9 mmol/L) on admission. Finally, Li et al. (28) observed in a much smaller sample of 132 patients that those with admission glucose >11 mmol/L were more likely to experience acute cardiac injury than those with admission glucose <11 mmol/L. By using a substantially larger sample, our study provides support for these findings by highlighting the heterogeneous relationship between blood glucose and complications, which demonstrated not only an intercomplication variability but also a varying association across the distribution of blood glucose levels.
We also provide evidence that associations between admission glucose and some cardiovascular and renal complications may be modified by age and preexisting diabetes status. While we are not aware of other studies that have explicitly investigated this association, a modifying role of age on the relationship between diabetes status and COVID-19 mortality and severity has been reported elsewhere and is in agreement with our findings, i.e., a stronger association in younger age-groups (29,30). Furthermore, in patients with COVID-19 and type 2 diabetes, the relationship between the underlying level of hyperglycemia and COVID-19 mortality was modified by age, with a steeper gradient of risk in patients age <70 years (8). Our finding of an attenuated risk of elevated glucose levels in older patients may reflect the absolute higher level of risk of complications in this age-group relative to younger adults in that elevated glucose levels in elderly individuals may not meaningfully affect the already higher rates of complications associated with older age, whereas other factors are likely to be more important. This is similar to findings related to the risk of mortality in obese patients with COVID-19, which was lower in those age >70 years (vs. younger) (31). Additionally, our finding of a potential modifying effect of preexisting diabetes status, with a higher likelihood of a poor prognosis observed in patients without a preexisting diagnosis of diabetes, has been reported elsewhere (32). This may reflect heightened glucose monitoring and a more timely implementation of specific admission treatment protocols (i.e., insulin) in patients with a preexisting diabetes diagnosis (33), resulting in improved glucose control in these patients compared with patients with high glucose levels but without a previous diabetes diagnosis. Strict glucose control has been shown to improve prognosis in both non–COVID-19 (34) and COVID-19 illness (35).
The mechanisms through which hyperglycemia may contribute to COVID-19–related cardiovascular and renal complications include dysregulation of the immune response, affecting the production of cytokines, such as interleukin 6, as well as altering the function of immune cells (35,36). Hyperglycemia also promotes glycosylation of the ACE2 receptor, which enables the binding of the SARS-CoV-2 virus to the host and therefore magnifies the extent of infection by SARS-CoV-2 (37). Hyperglycemia is also known to induce oxidative stress (38) and to have proinflammatory and prothrombotic effects (39,40). The consequences of the proinflammatory and oxidative stress effects of hyperglycemia and diabetes on the immune response, in the context of COVID-19, have been discussed comprehensively in a recent review (41). In terms of hypoglycemia, this has also been shown to induce proinflammatory and prothrombotic states (42,43), thus having the potential to affect the immune response because of a similar background of chronic inflammation as hyperglycemia (41). It has been shown that acute hypoglycemia can result in an increased expression of the CD40 receptor, which is involved in the inflammatory response (42) and may also increase hormonal adrenergic activity (44), further increasing the inflammatory response and risk of arrhythmia (45). As such, it has been speculated that hypoglycemia may represent a trigger mechanism for the cytokine storm during COVID-19 infection (46) and heart conduction abnormalities (47).
There may also be a bidirectional relationship between COVID-19 severity and hyperglycemia, with the resultant cytokine production following COVID-19 infection exacerbating insulin resistance or impairing insulin secretion (48,49). Furthermore, the binding of SARS-CoV-2 to ACE2 receptors, which are found to be expressed in pancreatic β-cells, provides a target for the virus to bind. Upon binding, the virus is able to enter and damage the pancreatic islets, resulting in defective insulin production (50). We tested this possibility by adjusting for oxygen saturation levels at admission as a proxy for COVID-19 severity. Upon adjustment, only a small degree of attenuation in the associations between admission glucose and complications were observed, suggesting a potentially independent contribution of admission glucose levels to the likelihood of experiencing an in-hospital cardiovascular or renal complication.
Strengths and Limitations
These data were collected prospectively across 302 facilities and represent a large heterogeneous proportion of people hospitalized for COVID-19 in the U.K., thus increasing the generalizability of our findings to the underlying population; robust estimates of short-term morbidity are important to provide to health care planners and policymakers. Other smaller or single-center studies have typically focused exclusively on patients who received critical care or on one type of complication and lack systematic approaches to data collection (51). This study includes all patients hospitalized with COVID-19. The large sample size enabled the testing of interactions by age, sex, ethnicity, obesity, and preexisting diabetes status, which may have been more difficult in a smaller sample given the relatively low prevalence of some of the ethnic groups/conditions.
We acknowledge a number of limitations. First, despite the relatively large sample size, a substantial proportion of the sample had missing glucose data. Possible explanations about why only some individuals had glucose measured include the selective blood sampling of patients with risk factors for diabetes (e.g., patients with obesity, patients of certain ethnic groups such as South Asian) or in those with a history of a previous diabetes diagnosis or other related preexisting disease. We compared the included and excluded samples across a number of key demographic variables and observed a greater number of patients with obesity, with preexisting diabetes, and of South Asian origin. As such, there may be some evidence that our sample overrepresents individuals more likely to exhibit poor glucose control compared with the full cohort and, thus, reduces the generalizability of the findings. Conversely, when comparing actual admission glucose levels in those included versus excluded, the mean of those included was lower than those excluded (145.8–156.6 mg/dL). Another explanation is that obtaining admission blood glucose levels could be driven by local protocols, which if random across hospitals and patients, is unlikely to introduce bias. Furthermore, because of a lack of data related to chronic glycemic control (i.e., HbA1c), we were unable to investigate the impact of a change in glucose control during admission and, thus, the role of stress hyperglycemia. It has been demonstrated that in non–COVID-19 illness, the stress hyperglycemia ratio may be a better predictor of acute kidney injury and in-hospital mortality and morbidity than admission glucose level (52). Second, because of a low prevalence of preexisting type 1 diabetes (2%), we combined type 1 and type 2 diabetes into a single diabetes variable. To explore whether this may have masked a heterogeneous effect modification by type of diabetes, we excluded patients with type 1 diabetes from the definition of preexisting diabetes; the results of the effect modification with glucose were almost identical across each of the complications studied. However, because both types of diabetes were based on a previous clinician diagnosis, our findings may still be obscured by cases of undiagnosed diabetes. Third, another limitation is that the data set focused on in-hospital complications during 28 days after the index admission for COVID-19 and did not contain longer-term outcome data. The prevalence of in-hospital complications was twice as high in the patients who remained hospitalized versus those discharged within 28 days, and the omission of longer-term follow-up and outcome data in patients who are likely to be at higher risk of subsequent complications and longer-term morbidity needs further study. Fourth, the complications that were captured were predefined by a pragmatic outbreak preparedness study protocol developed before the emergence of COVID-19 and may lack the granular details for certain aspects that could instead be collected in ad hoc cohort studies or trials. For example, we were unable differentiate between resuscitated and nonresuscitated cardiac arrest, which may have heterogeneous associations with admission glucose levels. All medical conditions, which were clinically defined following the diagnostic procedures deemed relevant by the health care professional, were extracted from clinical records and inputted from the local health professionals in charge of each individual’s care. Local investigators were able to enter other complications as free text, but this approach may have missed some important complications that were otherwise unexpected. Relatedly, obesity was defined by the ISARIC protocol using not only objective measures (e.g., BMI) but also subjective clinical judgment, which is likely to vary between clinicians and thus introduces bias into the definition of obesity. In addition, a lack of sociodemographic and lifestyle factors (e.g., smoking habits, physical activity) included in the study protocol means that the possibility of residual confounding biasing our estimates cannot be excluded. Finally, these data were collected from clinical practice, and patients did not undergo any additional tests to detect the presence of complications. Therefore, the estimates presented here are likely to be a conservative estimate of the true burden of complications. However, our estimates of in-hospital cardiovascular complications are higher than those reported in 3,011 patients with COVID-19 admitted to the hospital at the beginning of the pandemic (53). Given the exploratory nature of the study, we did not adjust for multiple comparisons, and we recommend that additional dedicated studies are conducted to confirm our findings.
Implications
Our study describes the increased risk of in-hospital cardiovascular and renal complications at both high and low glucose levels and in patients with and without diabetes. These findings highlight the importance of routine glucose screening on admission in order to implement individual treatment plans aimed at modifying any deleterious glucose levels.
It is likely that hospitalized patients with COVID-19 who survive after cardiovascular and renal complications will experience long-term morbidity. Given the prevalence of these complications in our study (30%), this indicates a potentially large future burden placed on the health care system. This is in addition to the substantial burden expected as a result of post-COVID syndrome (“long COVID”), which has been associated with increased risks of morbidity, hospital readmission, and mortality (54,55). As such, governments, policymakers, health care planners, and frontline health care workers should anticipate an increased burden placed on health and social care resources, which will be critical to support patients who survive COVID-19.
In conclusion, more than one-quarter of patients hospitalized with COVID-19 experienced a cardiovascular or renal complication. Increased odds of experiencing a cardiovascular or renal complication were observed at admission glucose levels indicative of both hypo- and hyperglycemia. For a number of complications, these associations were strengthened in younger patients and in patients without a preexisting diagnosis of diabetes. In light of findings of an increased odds of complications at both high and low glucose levels, admission glucose could be used as a marker for risk stratification of high-risk patients. Future research should evaluate interventions to determine optimal glycemic control by avoiding both hypo- and hyperglycemia in people with COVID-19.
T.N. and C.R. are joint first authors.
C.A.L. and K.K. are joint senior authors.
This article contains supplementary material online at https://doi.org/10.2337/figshare.19146053.
This article is part of a special article collection available at https://diabetesjournals.org/journals/collection/52/Diabetes-and-COVID-19.
A complete list of ISARIC4C Consortium investigators can be found in the supplementary material online.
Article Information
Acknowledgments. This work uses data provided by patients and collected by the National Health Service as part of their care and support (#DataSavesLives). The authors are grateful to the 2,648 frontline National Health Service clinical and research staff and volunteer medical students who collected these data in challenging circumstances and the generosity of the participants and their families for their individual contributions in these difficult times. The authors also acknowledge the support of Jeremy J. Farrar (Wellcome Trust, London, U.K.) and Nahoko Shindo (World Health Organization, Geneva, Switzerland).
Funding. This work was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre (BRC), NIHR Applied Research Collaboration–East Midlands (ARC-EM), the UK Research and Innovation Department of Health and Social Care (UKRI-DHSC) COVID-19 Rapid Response Rolling Call (grant MR/V020536/1), Health Data Research (HDR)-UK (grant HDRUK2020.138), the NIHR (award CO-CIN-01), the Medical Research Council (MRC) (grant MC_PC_19059), and by the NIHR Health Protection Research Unit (HPRU) in Emerging and Zoonotic Infections at the University of Liverpool.
The funder/sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Duality of Interest. K.K. is supported by the NIHR ARC-EM and T.Y. by the NIHR Leicester BRC. K.K. is director for the University of Leicester Centre for Black and Minority Ethnic Health, trustee of the South Asian Health Foundation, national NIHR ARC lead for ethnicity and diversity, member of Independent Scientific Advisory Group for Emergencies (SAGE), and chair of the SAGE subgroup on ethnicity and COVID-19. G.P.M. is supported by an NIHR Research Professorship (2017-08-ST2-007). M.G.S. is a member of HMG (Her Majesty's Government) SAGE COVID-19 and reports grants from the DHSC, NIHR, MRC, and NIHR HPRU in Emerging and Zoonotic Infections, University of Liverpool, during the conduct of the study. M.G.S. reports other support from Integrum Scientific LLC outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.N. wrote the first draft of the manuscript. T.N. and C.R. analyzed the data. T.N., C.R., C.A.L., and K.K. conceived of the study. T.Y., F.Z., C.L.G., Y.V.C., A.R., M.J.D., G.P.M., A.B., A.B.D., P.J.M.O., J.K.B., M.G.S., C.A.L., and K.K. reviewed the manuscript and provided important edits. C.A.L. and K.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.