While the adjustment of insulin is an established strategy to reduce the risk of exercise-associated hypoglycemia for individuals with type 1 diabetes, it is not easily feasible for those treated with ultra-long-acting basal insulin. The current study determined whether pre-exercise intake of fructose attenuates the risk of exercise-induced hypoglycemia in individuals with type 1 diabetes using insulin degludec.
Fourteen male adults with type 1 diabetes completed two 60-min aerobic cycling sessions with or without prior intake (30 min) of 20 g of fructose, in a randomized two-period crossover design. Exercise was performed in the morning in a fasted state without prior insulin reduction and after 48 h of standardized diet. The primary outcome was time to hypoglycemia (plasma glucose ≤3.9 mmol/L) during exercise.
Intake of fructose resulted in one hypoglycemic event at 60 min compared with six hypoglycemic events at 27.5 ± 9.4 min of exercise in the control condition, translating into a risk reduction of 87.8% (hazard ratio 0.12 [95% CI 0.02, 0.66]; P = 0.015). Mean plasma glucose during exercise was 7.3 ± 1.4 mmol/L with fructose and 5.5 ± 1.1 mmol/L in the control group (P < 0.001). Lactate levels were higher at rest in the 30 min following fructose intake (P < 0.001) but were not significantly different from the control group during exercise (P = 0.32). Substrate oxidation during exercise did not significantly differ between the conditions (P = 0.73 for carbohydrate and P = 0.48 for fat oxidation). Fructose was well tolerated.
Pre-exercise intake of fructose is an easily feasible, effective, and well-tolerated strategy to alleviate the risk of exercise-induced hypoglycemia while avoiding hyperglycemia in individuals with type 1 diabetes on ultra-long-acting insulin.
Introduction
Fructose, a monosaccharide with a delayed but enduring glycemic effect due to its distinct metabolism (1), was recently shown to have a beneficial metabolic impact in patients with type 1 diabetes performing exercise (2). Glycemic control in the context of exercise imposes high demands on individuals with type 1 diabetes. Physical exercise normally leads to an increase in peripheral glucose disposal and a consequent decrease in endogenous insulin secretion to maintain euglycemia (3). The lack of this autoregulation in individuals with type 1 diabetes predisposes to exercise-associated hypoglycemia due to inappropriately high exogenous insulin exposure (3). While the adjustment of insulin is a well-established strategy to reduce the risk of exercise-associated hypoglycemia (4), it is not easily feasible for a substantial number of patients, particularly in those treated with ultra-long-acting basal insulin (5). In these situations, the ingestion of additional carbohydrates may offer an alternative approach with glucose-based carbohydrates being frequently used in daily practice (4). However, the rapid glycemic effect of glucose may cause hyperglycemia and introduce substantial glycemic variability, thereby potentially deteriorating glucose control. Fructose, due to its metabolic properties, may offer an alternative carbohydrate supplementation strategy.
Here we present a proof-of-principle study building upon previous metabolic experiments and extending the investigation toward a potential clinical role of exclusive fructose consumption. We hypothesized that pre-exercise intake of 20 g of fructose would substantially delay exercise-induced hypoglycemia, while avoiding hyperglycemia in adults with type 1 diabetes treated with the ultra-long-acting insulin degludec.
Research Design and Methods
Study Design and Participants
In this open-label, randomized, single-center crossover study, we recruited male adults with type 1 diabetes using insulin degludec (Tresiba; Novo Nordisk A/S, Bagsværd, Denmark) as basal insulin and attending the University Hospital Bern outpatient diabetes clinic. Insulin degludec was chosen because it is currently the basal insulin analog with the longest duration of action (>42 h) (6). Inclusion criteria were type 1 diabetes (defined according to World Health Organization criteria) for ≥2 years, age between 18 and 45 years, HbA1c ≤8.0% (≤64 mmol/mol), use of insulin degludec for at least 3 months, and regular exercise (≥30 min of moderate-to-vigorous aerobic activity greater than or equal to three times weekly). Exclusion criteria were established nephropathy, neuropathy, or proliferative retinopathy; total daily insulin dose ≥2.0 units/kg; hypoglycemia unawareness as determined by Gold score ≥4 based on prestudy clinical records (7) or a recent (≤6 months) episode of severe hypoglycemia; or known intolerance to fructose. Because of the considerable impact of sex hormones on exercise-related fuel metabolism, female participants were excluded from this study (8–10).
The study was approved by the Ethics Committee Bern, Switzerland, and registered on ClinicalTrials.gov (NCT03497260). All participants provided written informed consent before the start of study-related procedures.
Randomization
Participants were randomly assigned (1:1) to receive fructose first, followed by water, or vice versa, using computer-generated block randomization. Participants and investigators were not masked to treatment allocation.
Baseline Assessment
After screening, all participants underwent a fructose tolerance test (20-g d-fructose; Fluka Analytic, Sigma-Aldrich, Buchs, Switzerland), measurement of body composition (Nutriguard-MS; Data Input GmbH, Pöcking, Germany), and an incremental cardiopulmonary exercise test (CPET) to determine the workload of the experimental visits. The CPET was performed on an electronically braked cycle ergometer with breath-by-breath spirometry (ergoselect 100; ergoline GmbH, Bitz, Germany). Expired respiratory gases were monitored continuously throughout the test via an open-circuit, automated, indirect calorimetry system (Quark CPET; Cosmed, Rome, Italy). The test ended when subjects reached volitional exhaustion. VO2max was defined as the highest 30-s average value of VO2 and was expressed relative to body weight (mL/kg/min). Information about the participant’s level of activity was obtained using the International Physical Activity Questionnaire (IPAQ)–short form, providing a MET-minutes per week (11).
Pre- and Postexercise Procedures
During the 48 h before the experimental visits, participants were asked to avoid alcohol, caffeine, and strenuous exercise. Participants were fitted with the Dexcom G6 continuous glucose monitoring system (DexCom Inc, San Diego, CA) for 48 h before and 24 h after the experimental visits and were required to stay on their usual basal insulin regimen. During the same period, participants adhered to a weight-maintenance diet (40–45% carbohydrate, 20% protein, and 35–40% fat), kept a food diary before the first visit, and were asked to match their food intake before the second visit. Experimental visits were rescheduled if the participants experienced hypoglycemia (sensor glucose <3.0 mmol/L) within 12 h before testing, to avoid that antecedent hypoglycemia influences glucoregulatory responses to subsequent exercise (12).
Experimental Visits
Participants reported to the clinical research facility in the morning after an overnight fast. An intravenous cannula was inserted into the antecubital vein for frequent blood sampling. Blood glucose was corrected if outside the predefined range (5.0–8.0 mmol/L) using intravenous insulin aspart (NovoRapid; Novo Nordisk) or oral glucose (dextrose tablets) according to individual correction factors. Once glycemia was stable, participants received 20 g of fructose dissolved in 200 mL of tap water or 200 mL of tap water without fructose 30 min before exercise. Plasma glucose and lactate were determined every 5 min from venous blood using the Biosen C-line analyzer (IGZ Instruments AG, Zürich, Switzerland) from the fructose/water intake until 30 min after exercise. Until exercise onset, participants remained in a sitting position and underwent pre-exercise blood sampling and a 5-min collection of respiratory gas (VCO2 and VO2) to calculate substrate oxidation using nonprotein respiratory quotient equations (13). The intensity of the 60-min cycling session on an electronically braked leg-cycle ergometer was set to 50% of the maximal power reached during the incremental cycling test. Participants were asked to maintain a pedal-rate of 60–70/min. During exercise, respiratory gases (VCO2 and VO2) were collected between the 15th and 20th minute of exercise. Heart rate was continuously monitored (Cosmed heart rate probe; Cosmed), and perceived exertion was assessed every 10 min using the Borg scale (14). The exercise was stopped at 60 min of exercise or when hypoglycemia occurred, defined as plasma glucose ≤3.9 mmol/L. A hypoglycemic event was corrected with 15 g of rapid-acting carbohydrates. If plasma glucose level remained ≤3.9 mmol/L after 15 min, the oral dose was repeated.
End Points
The primary end point was time to hypoglycemia (plasma glucose ≤3.9 mmol/L) during the 60 min of exercise. Secondary end points were mean glucose and lactate levels before exercise, during exercise (measured from start of the exercise until 60 min or hypoglycemia occurrence), and during 30 min after exercise; substrate oxidation before and during exercise; mean heart rate; and level of perceived exertion during exercise. Safety end points were severe hypoglycemic events, significant ketonemia (>3.0 mmol/L), fructose intolerability, and other adverse events.
Statistical Analysis
The power calculation was based on the assumption that a single load of 20 g of fructose provides 10–12 g of glucose (15), thereby postponing exercise-related hypoglycemia by a minimum of 15 min (16). A sample size of 14 subjects was deemed necessary to detect a difference in the time to hypoglycemia of 15 min between arms with a power of 80% for paired survival data at a 5% significance level. We planned to recruit up to 16 participants, aiming for 14 participants with complete data.
The time to hypoglycemia was compared using a Cox proportional hazards regression model with the study period entered into the model and considering subjects as clusters to account for the repeated design. Secondary end points were compared using linear mixed models with intervention and period entered as fixed effects and subjects as random effects. In case of nonnormality of the residuals, data were transformed appropriately. Diagnostic plots of all performed models were visually inspected with regard to satisfaction of underlying statistical assumptions, such as normal distribution of residuals, homoscedasticity, and influential data points. A generalized linear mixed effect model with Poisson distribution was used to compare the number of postexercise hypoglycemic events (17).
Results are reported as mean ± SD or median (interquartile range) for each condition (fructose, water). Data obtained after hypoglycemia correction are not considered in the reported means. Statistical significance was set at 5% for all comparisons. Analyses were performed with the software R, version 3.6.0 (18).
Results
Study Participants
Between 4 January and 6 September 2019, we recruited 15 participants (Supple‐mentary Fig. 1). One subject prematurely stopped the exercise during the second visit and was excluded from the final analysis, which included complete data from 14 participants.
Participant characteristics are shown in Table 1. Participants were well controlled (HbA1c 6.9 ± 0.7%; 52 ± 7 mmol/mol), had a diabetes duration of 17.5 ± 10.5 years, and used a total daily insulin dose of 0.6 ± 0.2 units/kg, of which 48.7 ± 9.9% was administered as insulin degludec. On the basis of the IPAQ-short form questionnaire, participants were moderately to highly active (MET-min per week = 3,788 ± 3,573), and CPET suggested average fitness (VO2max 40.0 ± 8.6 mL/min/kg).
Participant characteristics
| Characteristics . | Value (n = 14) . |
|---|---|
| Age (years) | 31.1 ± 8.2 |
| Body weight (kg) | 82.2 ± 11.4 |
| BMI (kg/m2) | 25.8 ± 3.3 |
| HbA1c (%; mmol/mol) | 6.9 ± 0.7 (52 ± 7) |
| Diabetes duration (years) | 17.5 ± 10.5 |
| Total daily insulin dose (units/kg) | 0.6 ± 0.2 |
| % insulin degludec to daily insulin dose | 48.7 ± 9.9 |
| Fat mass (%) | 16.4 ± 7.7 |
| VO2max (mL/min/kg) | 40.0 ± 8.6 |
| Power at VO2max (W) | 301.5 ± 80.7 |
| Characteristics . | Value (n = 14) . |
|---|---|
| Age (years) | 31.1 ± 8.2 |
| Body weight (kg) | 82.2 ± 11.4 |
| BMI (kg/m2) | 25.8 ± 3.3 |
| HbA1c (%; mmol/mol) | 6.9 ± 0.7 (52 ± 7) |
| Diabetes duration (years) | 17.5 ± 10.5 |
| Total daily insulin dose (units/kg) | 0.6 ± 0.2 |
| % insulin degludec to daily insulin dose | 48.7 ± 9.9 |
| Fat mass (%) | 16.4 ± 7.7 |
| VO2max (mL/min/kg) | 40.0 ± 8.6 |
| Power at VO2max (W) | 301.5 ± 80.7 |
Data are presented as mean ± SD.
Pre-Exercise Assessments
In the 48 h before the experimental visits, participants consumed 264 ± 23 g of carbohydrate, 97 ± 9 g of fat, and 138 ± 11 g of protein per day (2,489 ± 254 kcal). Two experimental visits were postponed due to antecedent hypoglycemia (sensor glucose <3.0 mmol/L occurring <12 h before the test) according to the protocol. Glucose control and variability did not significantly differ between the study arms (Supplementary Table 1).
Experimental Visits
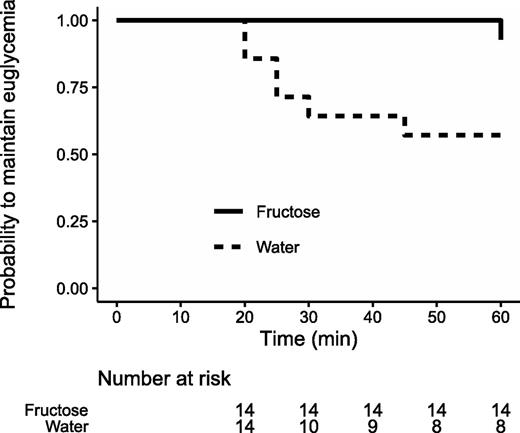
Hypoglycemia occurred in one participant (after 60 min of exercise) after fructose ingestion compared with six participants (after 27.5 ± 9.4 min of exercise) in the control condition, translating into a 87.8% risk reduction with fructose (hazard ratio 0.12; 95% CI 0.02, 0.66; P = 0.015) (Fig. 1). Plasma glucose and lactate values before and during exercise are shown in Table 2. Plasma glucose at the time of fructose/water intake did not statistically differ between the conditions (6.5 ± 0.7 mmol/L with fructose vs. 6.3 ± 0.1 mmol/L with water, P = 0.51). Fructose intake 30 min before exercise gradually increased glycemia over 49.5 min (range 44.3–58.8 min) by a maximum of 1.8 ± 1.1 mmol/L. Mean glucose during exercise was significantly lower in the control condition than after the intake of fructose (5.5 ± 1.1 vs. 7.3 ± 1.4 mmol/L, P < 0.001). The exercise-induced decrease in glycemia did not significantly differ between the conditions (−1.0 ± 1.7 mmol/L with fructose vs. −1.5 ± 1.2 mmol/L in the control group, P = 0.44) (Table 2). The dynamics of the difference between glucose values after fructose versus water intake during the exercise period are illustrated in Supplementary Fig. 2. Individual and mean glucose profiles during the experimental visits are illustrated in Fig. 2.
Kaplan-Meier curves indicating time to hypoglycemia during the 60-min exercise session. Straight line denotes fructose intake, and dotted line indicates water intake.
Kaplan-Meier curves indicating time to hypoglycemia during the 60-min exercise session. Straight line denotes fructose intake, and dotted line indicates water intake.
Plasma glucose and lactate levels before, during, and after exercise
| . | Fructose (n = 14) . | Water (n = 14) . | Paired difference (95% CI) (n = 14) . | P value . |
|---|---|---|---|---|
| Glucose at fructose/water intake (mmol/L)# | 6.5 ± 0.7 | 6.3 ± 1.0 | 0.2 (–0.4, 0.8) | 0.51 |
| Glucose at start of exercise (mmol/L) | 7.2 ± 1.3 | 6.2 ± 1.3 | 1.6 (0.4, 1.0) | 0.003 |
| Mean glucose during exercise (mmol/L) | 7.3 ± 1.4 | 5.5 ± 1.1 | 1.9 (1.4, 2.3) | <0.001 |
| Mean glucose over 30 min after exercise (mmol/L)‡ | 6.4 ± 1.9 | 5.3 ± 1.1 | 1.2 (0.3, 2.2) | 0.014 |
| Δ glucose(−30 min to end of exercise) (mmol/L)# | −0.2 ± 1.8 | −1.5 ± 1.4 | 1.5 (0.3, 2.6) | 0.019 |
| Δ glucose(−30 min to 0 min) (mmol/L)# | 0.9 ± 0.7 | −0.1 ± 0.4 | 1.0 (0.8, 1.3) | <0.001 |
| Δ glucose(exercise) (mmol/L) | −1.0 ± 1.7 | −1.5 ± 1.2 | 0.4 (–0.7, 1.6) | 0.44 |
| Lactate at fructose/water intake (mmol/L)# | 0.7 ± 0.2 | 0.8 ± 0.2 | −0.1 (0.03, 0.2) | 0.017 |
| Lactate at start of exercise (mmol/L) | 1.2 ± 0.3 | 0.8 ± 0.3 | 0.5 (0.3, 0.7) | <0.001 |
| Mean lactate during exercise (mmol/L) | 2.5 ± 1.1 | 2.4 ± 1.0 | 0.2 (–0.2, 0.6) | 0.32 |
| Δ lactate(−30 min to 0 min) (mmol/L)# | 0.5 ± 0.2 | −0.1 ± 0.1 | 0.6 (0.5, 0.8) | <0.001 |
| Δ lactate(exercise) (mmol/L) | 1.0 ± 1.2 | 1.4 ± 1.0 | −0.3 (–0.8, 0.1) | 0.14 |
| . | Fructose (n = 14) . | Water (n = 14) . | Paired difference (95% CI) (n = 14) . | P value . |
|---|---|---|---|---|
| Glucose at fructose/water intake (mmol/L)# | 6.5 ± 0.7 | 6.3 ± 1.0 | 0.2 (–0.4, 0.8) | 0.51 |
| Glucose at start of exercise (mmol/L) | 7.2 ± 1.3 | 6.2 ± 1.3 | 1.6 (0.4, 1.0) | 0.003 |
| Mean glucose during exercise (mmol/L) | 7.3 ± 1.4 | 5.5 ± 1.1 | 1.9 (1.4, 2.3) | <0.001 |
| Mean glucose over 30 min after exercise (mmol/L)‡ | 6.4 ± 1.9 | 5.3 ± 1.1 | 1.2 (0.3, 2.2) | 0.014 |
| Δ glucose(−30 min to end of exercise) (mmol/L)# | −0.2 ± 1.8 | −1.5 ± 1.4 | 1.5 (0.3, 2.6) | 0.019 |
| Δ glucose(−30 min to 0 min) (mmol/L)# | 0.9 ± 0.7 | −0.1 ± 0.4 | 1.0 (0.8, 1.3) | <0.001 |
| Δ glucose(exercise) (mmol/L) | −1.0 ± 1.7 | −1.5 ± 1.2 | 0.4 (–0.7, 1.6) | 0.44 |
| Lactate at fructose/water intake (mmol/L)# | 0.7 ± 0.2 | 0.8 ± 0.2 | −0.1 (0.03, 0.2) | 0.017 |
| Lactate at start of exercise (mmol/L) | 1.2 ± 0.3 | 0.8 ± 0.3 | 0.5 (0.3, 0.7) | <0.001 |
| Mean lactate during exercise (mmol/L) | 2.5 ± 1.1 | 2.4 ± 1.0 | 0.2 (–0.2, 0.6) | 0.32 |
| Δ lactate(−30 min to 0 min) (mmol/L)# | 0.5 ± 0.2 | −0.1 ± 0.1 | 0.6 (0.5, 0.8) | <0.001 |
| Δ lactate(exercise) (mmol/L) | 1.0 ± 1.2 | 1.4 ± 1.0 | −0.3 (–0.8, 0.1) | 0.14 |
Data are presented as mean ± SD or mean (95% CI).
n = 13 for fructose as well as for the paired difference, due to missing blood sampling at time −30 min in a subject.
n = 13 for fructose, 8 for water, and 8 for the paired difference. Fructose/water intake occurred at time −30 min, start of exercise at 0 min, and end of exercise at 60 min or in the event of hypoglycemia.
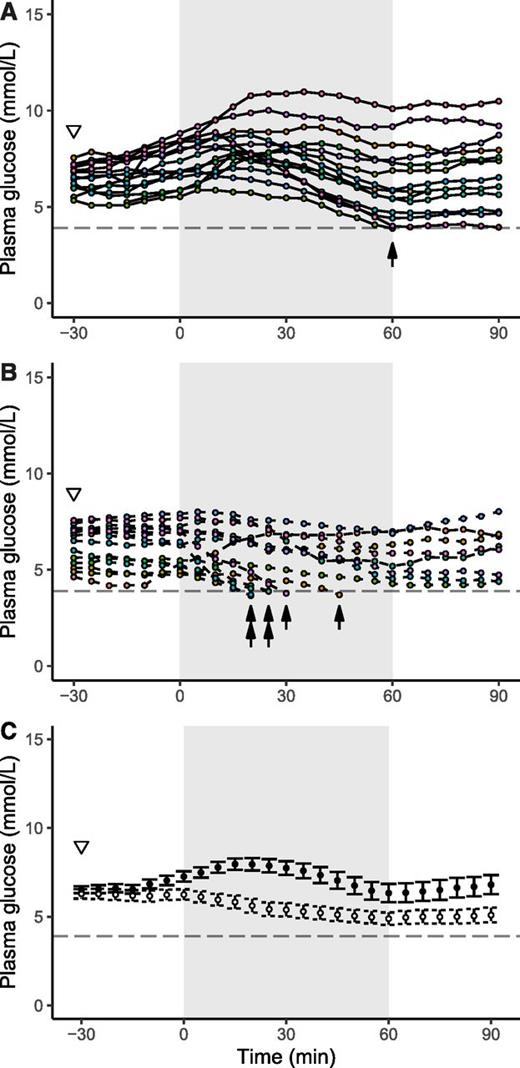
Individual curves of plasma glucose with fructose intake (n = 14) (A) and water intake (n = 14) (B) as well as mean glucose concentration (n = 14 in each group, with last observation carried forward for participants experiencing hypoglycemia) (C). Black dots represent fructose intake, and white dots represent water intake. Colors denote different participants. Time of fructose/water intake (−30 min) is indicated by a white triangle (▿). The exercise period is denoted by gray shading. Hypoglycemic events are indicated by arrows. In C, data are presented as mean ± SE.
Individual curves of plasma glucose with fructose intake (n = 14) (A) and water intake (n = 14) (B) as well as mean glucose concentration (n = 14 in each group, with last observation carried forward for participants experiencing hypoglycemia) (C). Black dots represent fructose intake, and white dots represent water intake. Colors denote different participants. Time of fructose/water intake (−30 min) is indicated by a white triangle (▿). The exercise period is denoted by gray shading. Hypoglycemic events are indicated by arrows. In C, data are presented as mean ± SE.
Fructose intake increased lactate levels in the resting period (lactate at start of exercise: 1.2 ± 0.3 vs. 0.8 ± 0.3 mmol/L, P < 0.001), whereas no significant difference between the conditions was observed in mean level during exercise (2.5 ± 1.1 mmol/L with fructose vs. 2.4 ± 1.0 mmol/L with water, P = 0.14).
Fructose intake increased carbohydrate oxidation (64.4 ± 16.2% vs. 43.4 ± 19.9%, P < 0.001) and lowered fat oxidation (35.6 ± 16.3% vs. 56.6 ± 19.4%, P < 0.001) during resting condition, whereas substrate oxidation rates were similar during exercise (carbohydrate: 81.7 ± 13.6% vs. 83.9 ± 16.4%, P = 0.61; fat: 18.3 ± 13.6% vs. 16.1 ± 16.4%, P = 0.61, for fructose and water, respectively). Cardiorespiratory parameters and perceived levels of exertion did not significantly differ between the conditions (all P > 0.05) (Supplementary Table 2).
Postexercise Assessment
Mean plasma glucose over the 30-min postexercise period was 6.4 ± 1.9 mmol/L in the fructose and 5.3 ± 1.1 mmol/L in the control condition (P = 0.014) (Table 2), without any hypoglycemic event in either group. Glucose control based on continuous glucose monitoring did not differ for the 24 h after the exercise period (Supplementary Table 1).
Safety Events
Fructose intake was well tolerated without any participant experiencing discomfort or other side effects. No adverse or serious adverse events, episodes of severe hypoglycemia, or episodes of hyperglycemia with ketosis occurred.
Conclusions
The present proof-of-principle study demonstrates that the consumption of 20 g of fructose before a 60-min aerobic cycling session in adults with type 1 treated with insulin degludec attenuates the risk of exercise-induced hypoglycemia.
Hypoglycemia during exercise presents a major challenge to individuals with type 1 diabetes, particularly in settings where pre-exercise insulin adjustment is not feasible or not effective. Several strategies have been elaborated to mitigate the risk of exercise-induced hypoglycemia. For example, short sprints before or during aerobic exercises or resistance training have shown a glycemia-stabilizing effect (19,20). However, the most frequently adopted strategy in daily life is the consumption of extra carbohydrates. In this context, glucose, either as simple or complex carbohydrates, is commonly consumed. For a 60-min aerobic activity without prior insulin dose adjustment, 15–30 g of extra carbohydrates are usually recommended (21). Although the intake of 20 g of glucose (e.g., as dextrose tablets) is effective in preventing exercise-induced hypoglycemia, it often leads to development of hyperglycemia due to the immediate glycemic impact (22). Subcutaneous administration of glucagon, another means to prevent or treat exercise-induced hypoglycemia, similarly induces a brisk increase in glycemia, although more-controlled stabilizing effects can be achieved with minidoses delivered by injection (22) or via dual hormone closed-loop systems (23). Regardless of the cause, there is evidence that exercising in hyperglycemia is not advisable due to a greater reliance on carbohydrate utilization, resulting in more pronounced declines in glycemia (24,25) and prevention of the shift toward increased lipid utilization (26).
Fructose, as an alternative to glucose, lacks the rapid glycemic impact due to an entirely distinct metabolism. It is absorbed via its specific transporter (GLUT5) (27) and is extracted by splanchnic tissues where it is converted to glucose, lactate, and de novo synthesized fatty acids by a specific set of enzymes (1). These ubiquitous energy substrates can either be locally stored or fuel the peripheral organs, depending on the metabolic context (2,28,29). We and others have previously explored the use of fructose in combination with glucose for carbohydrate supplementation in exercising adults with type 1 diabetes and have found beneficial effects in terms of both glycemic stability and substrate oxidation (2,30,31). The idea of using a single load of pure fructose in the current study was inspired from the incentive to further enhance the convenience of supplementation (avoidance of repeated doses), while eliminating carbohydrate-induced blood glucose spikes and dysglycemia. We observed that fructose intake 30 min before exercise gradually increased plasma glucose over 50 min by ∼2 mmol/L, thereby effectively preventing hypoglycemia without inducing unfavorable plasma glucose spikes. A recently published study using stable isotopes demonstrated that glucose requirements (intravenous glucose) during aerobic exercise under basal insulinemic conditions only start to rise after 30 min (25), which concurs with the timing of hypoglycemia observed in the control condition of the current study. This observation further suggests that scheduling fructose intake immediately before exercise rather than 30 min earlier as done in the current study, would provide an even more favorable match with an exercise-induced increase in glucose requirements, particularly for sessions of longer duration. In line with our previous findings, fructose did not alter net carbohydrate oxidation during exercise, indicating that oxidation of fructose-derived glucose and lactate spares the utilization of endogenous glucose (plasma glucose from hepatic glycogen and muscle glycogen) (2).
The quality and quantity of carbohydrates used in the current study (20 g of fructose) differs from recommendations in current guidelines that recommend intake of glucose supplements (i.e., tablets, gels, and so on), starch, or sucrose-containing snacks (4,21). While we acknowledge that the present data reflect findings of a proof-of-principle study, exclusively, our study provides evidence that the sustained release profile of fructose may optimally match the course of basal exercise-induced requirements. Improved matching of energy provisions with requirements may translate into lower amounts of exogenous carbohydrates requested to uphold normoglycemia throughout exercise, which is also relevant from a weight-managing perspective for patients.
While excess intake of fructose as part of a hypercaloric diet, due to its well-established lipogenic properties, may lead to adverse metabolic effects, such as hepatic fat accumulation and insulin resistance, these complications have not been substantiated in active and exercising individuals (32). Thus, we consider the risks attributable to moderate amounts of fructose consumption in the context of exercise in individuals with type 1 diabetes as low. Of note, the intake of a higher amount of fructose as a single dose may be associated with gastrointestinal side effects due to malabsorption (33). Hence, for exercise of longer duration, a repetitive intake of a 20-g dose may be the preferred strategy rather than increasing the pre-exercise load.
The strengths of this study are its clinical focus, the randomized crossover design, and the standardization of the pre-exercise procedures. We also acknowledge limitations. The study excluded females due to the well-known effects of sex-related differences in hormonal responses, exercise-related fuel metabolism, and fructose utilization (8–10,34). Thus, further studies are warranted to investigate the metabolic effects of fructose consumption in females with type 1 diabetes engaging in exercise. The study intervention was carried out in the morning, which may not reflect the most common time of day of recreational exercise performance given the well-established circadian differences in insulin sensitivity (35–37). Since we studied adults on insulin degludec treatment exclusively, our findings cannot be unrestrictedly extrapolated to individuals treated with different types of insulin replacement and dosing regimens. Finally, we want to strengthen the notion that while the findings of the current study corroborate the potential of fructose to cover basal energy requirements during exercise, they do not interfere with the clear role of glucose as a rapid means to correct hypoglycemia. Since the current study aimed at a proof of the concept that fructose may be used to stabilize exercise-associated glycemia, an active comparator was not included. However, direct comparison of fructose and glucose should now be tested in a subsequent full-scale study.
In conclusion, the current study suggests that fructose intake before exercise in individuals with type 1 diabetes is a feasible, effective, and well-tolerated strategy to attenuate the risk of exercise-induced hypoglycemia.
Clinical trial reg. no. NCT03497260, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.12302948.
L.B. and C.S. contributed equally as last authors.
C.K. and D.H. contributed equally as first authors.
Article Information
Acknowledgments. The authors thank the study volunteers for their participation, the study nurses from the Department of Nephrology and Hypertension of the University Hospital Bern for logistical support, and Michèle Monnard (Department of Anaesthesiology and Pain Medicine, University Hospital Bern) for assistance with data management.
Funding. This study was supported in part by the UDEM Scientific Fund.
Duality of Interest. This work is an investigator-initiated clinical study supported in part by the Investigator Sponsored Studies program of Novo Nordisk A/S, Denmark. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.K. screened and enrolled participants, conducted the study visits, collected and prepared the data, and contributed to the manuscript writing. D.H. contributed to the collection of the data, analyzed the data, and produced the display items. C.I.L. contributed to the recruitment, conductance of the experiments, and data collection. C.T.N. and L.B. contributed to the statistical analysis. A.M. contributed to the protocol writing. L.B. and C.S. designed the study, interpreted the findings, and wrote the manuscript. All authors critically reviewed the manuscript. L.B. and C.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in oral form at the Annual Meeting of the Swiss Society of Endocrinology and Diabetology, Bern, Switzerland, 15 November, 2019.