To evaluate feasibility, safety, and efficacy of overnight closed-loop insulin delivery in free-living youth with type 1 diabetes.
Overnight closed loop was evaluated at home by 16 pump-treated adolescents with type 1 diabetes aged 12–18 years. Over a 3-week period, overnight insulin delivery was directed by a closed-loop system, and on another 3-week period sensor-augmented therapy was applied. The order of interventions was random. The primary end point was time when adjusted sensor glucose was between 3.9 and 8.0 mmol/L from 2300 to 0700 h.
Closed loop was constantly applied over at least 4 h on 269 nights (80%); sensor data were collected over at least 4 h on 282 control nights (84%). Closed loop increased time spent with glucose in target by a median 15% (interquartile range −9 to 43; P < 0.001). Mean overnight glucose was reduced by a mean 14 (SD 58) mg/dL (P < 0.001). Time when glucose was <70 mg/dL was low in both groups, but nights with glucose <63 mg/dL for at least 20 min were less frequent during closed loop (10 vs. 17%; P = 0.01). Despite lower total daily insulin doses by a median 2.3 (interquartile range −4.7 to 9.3) units (P = 0.009), overall 24-h glucose was reduced by a mean 9 (SD 41) mg/dL (P = 0.006) during closed loop.
Unsupervised home use of overnight closed loop in adolescents with type 1 diabetes is safe and feasible. Glucose control was improved during the day and night with fewer episodes of nocturnal hypoglycemia.
Introduction
Achievement of tight glycemic control in type 1 diabetes is limited by hypoglycemia (1), particularly overnight when the sympathoadrenal response to falling blood glucose concentration is blunted, reducing warning symptoms and arousal from sleep (2). The risk of severe hypoglycemia is the most feared adverse event among young people with type 1 diabetes and their families, contributing to four in five youth aged 13–18 years failing to meet the International Society for Pediatric and Adolescent Diabetes glycemic control target of HbA1c <7.5% (58.5 mmol/mol) (3).
Technology-assisted therapeutic approaches such as insulin pump therapy have led to improved glycemic control and reduced risk of severe hypoglycemia (4). Continuous glucose monitoring enables users to view real-time glucose readings with alarms for impending hypo- or hyperglycemia facilitating appropriate changes in insulin delivery (5). When sensor wear is regular, continuous glucose monitoring can improve glucose control, but poor compliance is particularly prevalent in adolescents (6). Sensor-augmented insulin pump therapy combining continuous glucose monitoring with insulin pump delivery further improves glycemic control but does not reduce risk of hypoglycemia (7) unless combined with threshold-based insulin pump interruptions (8,9).
Closed-loop systems differ from conventional and threshold-suspend sensor-augmented pump therapy through the use of a control algorithm which automatically reduces and increases subcutaneous insulin delivery according to sensor glucose levels. Preliminary short-duration studies (up to 2 days in carefully controlled hospital settings) suggest the potential to safely improve glucose control with the model predictive control algorithm in youth (10,11), adults (12–14), and pregnant women (15) and with proportional-integral-derivative (16,17), fuzzy logic (18), and glucagon coadministration approaches (19). Outside hospital settings, two single-night transitional studies have been conducted, involving a diabetes camp and hotel setting, both with intensive on-site and/or telemonitoring supervision (20,21). An interim analysis of a 4-night home overnight closed-loop study using telemonitoring has been reported (22).
There has been no previous prolonged evaluation of closed loop under free-living conditions. We hypothesized that unsupervised home use of overnight closed loop was safe and feasible and would improve glucose control compared with sensor-augmented pump therapy in young people with type 1 diabetes.
Research Design and Methods
We carried out an open-label, randomized, crossover study to compare sensor- augmented pump therapy with and without overnight closed-loop insulin delivery in adolescents with type 1 diabetes. The study was performed in real-life conditions, with unrestricted diet and normal school and sporting activities and without telemonitoring or continuous supervision. Participants aged ≥16 years and parents or guardians of participants aged ≤16 years signed informed consent; written assent was obtained from minors. The study was approved by the Local Research Ethics Committee.
Setting and Subjects
The study was conducted at home with participants recruited from Pediatric Diabetes Clinics at Addenbrooke’s Hospital, Cambridge, U.K., and University College Hospital, London, U.K., from July 2012 to March 2013. Inclusion criteria were type 1 diabetes, age 12–18 years, >1 year from diagnosis or confirmed C-peptide–negative, insulin pump therapy for at least 3 months, four or more fingerstick glucose measurements per day, and HbA1c ≤10% (86 mmol/mol). Exclusion criteria included established nephropathy, neuropathy, or proliferative retinopathy, total daily insulin dose ≥2.0 U/kg, regular use of continuous glucose monitoring within 1 month prior to enrollment, severe visual or hearing impairment, pregnancy, or breastfeeding.
Study Design
In an open-label, randomized, two-period crossover study design, participants underwent two 21-day periods of sensor-augmented pump therapy with and without overnight closed loop (Fig. 1). During both 21-day periods, participants used the study pump and real-time continuous glucose-monitoring devices, the latter calibrated according to the manufacturer’s instructions. Standard hypoglycemia and hyperglycemia treatment guidelines were followed. The sensor glucose alarm threshold was set at 63 mg/dL. Rapid-acting insulin analog Aspart (Novo Nordisk, Bagsvaerd, Denmark) was used.
During one 21-day period, randomly assigned, overnight insulin delivery was directed by a closed-loop algorithm. During the 2- to 3-week washout, participants used their standard pump and discontinued continuous glucose monitoring. Randomization assignment was unblinded, but allocation between treatment sequences was concealed to the study staff until after randomization, which occurred the day prior to the first intervention. Randomization used permuted block-four approach.
Training and Supervision
After enrollment, participants were trained on the specific features of the study insulin pump (Dana R Diabecare; Sooil, Seoul, South Korea) and continuous glucose-monitoring (FreeStyle Navigator; Abbott Diabetes Care, Alameda, CA) devices (23). Over a 2–4-week run-in phase, participants used the study pump and continuous glucose monitor and were required to collect at least 8 days of sensor glucose readings to pass the run-in phase assessment. The Navigator receiver was modified during the run-in phase to record sensor glucose levels but not to display them. Data were used to optimize insulin pump therapy.
On the first evening of closed loop, training was provided in the participants’ homes on operation, including initiation and discontinuation of the closed-loop system. Participants were instructed to start closed loop in the evening and to stop before breakfast. One or two members of the study team were accommodated close to participants’ homes during the first closed-loop night to alleviate potential safety concerns and to supervise closed-loop stopping the next morning. No further supervision took place over the following 20 nights, and telemonitoring was not used.
Closed-Loop System
The Florence closed-loop system (24) comprises a model predictive control algorithm residing on a small laptop, which is linked by cable to the sensor receiver and controls the study pump via wireless communication.
Every 12 min, the treat-to-target algorithm calculated a new insulin infusion rate, which is automatically set on the study pump (Supplementary Fig. 2). The calculations use a compartment model of glucose kinetics (25) describing the effect of rapid-acting insulin and the carbohydrate content of meals on glucose levels. Carbohydrate content was downloaded automatically from the study pump. Insulin delivery history was also downloaded including manually instructed insulin boluses. The algorithm was initialized using preprogrammed basal insulin delivery downloaded from the study pump. Additionally, the participant’s weight and total daily insulin dose were entered at setup. Algorithm version 0.3.24 with interface version 1.0.7 was used (University of Cambridge).
Safety Precautions During Closed Loop
Participants performed a calibration check before their evening meal. If sensor glucose was above fingerstick glucose by >54 mg/dL, the continuous glucose monitor was recalibrated, and calibration check was repeated before starting closed loop. These instructions mitigated risk of sensor error and were tested in silico (26) using the validated Cambridge simulator (27).
If sensor glucose reading outputs became unavailable or in case of other failures, the subject’s usual insulin delivery rate automatically restarted within 30–60 min. These measures limited the risk of insulin under- and overdelivery (26). The algorithm included rules that limited maximum insulin infusion and suspended insulin delivery if glucose was ≤77 mg/dL or when glucose was rapidly decreasing.
Assays
Baseline random C-peptide was measured by an immunometric assay (Siemens Healthcare Diagnostics, Frimley, U.K.).
Sample Size
Based on 24–36 h in-patient pilot (10,12,28), we anticipated that overnight closed-loop insulin delivery could increase the percentage nighttime glucose was between 70 and 144 mg/dL by a mean 24% (SD 29%). We calculated that 16 participants would provide 80% power at the 5% level of significance to detect such difference between sensor-augmented pump therapy and overnight closed-loop insulin delivery.
Statistical Analysis
The analysis plan was agreed in advance. The primary outcome was the time when glucose was in the target range (70–144 mg/dL) between 2300 and 0700 h. Secondary outcomes included mean glucose, time when glucose was <70 mg/dL (hypoglycemia), number of nights when glucose was <63 mg/dL for ≥20 min, time when glucose was >144 mg/dL (hyperglycemia), and insulin delivery. We estimated glycemic variability by the coefficient of variation of glucose during nights and between nights. Previously, we showed that Navigator sensor glucose is acceptable to estimate glucose mean and variability but may overestimate benefit of closed loop (29). We corrected for this bias resulting from simultaneous use of sensor glucose to both direct insulin delivery and assess outcomes by using a conservative stochastic transformation of threshold metrics assessing time glucose was in, below, and above target (29). Stochastic transformation has been shown to provide an unbiased estimate of time when glucose is in target and below target, making it suitable for assessment of closed loop in outpatient settings.
We included in the primary and secondary analyses evaluable nights comprising at least 4 h of sensor data (control group) or 4 h of uninterrupted closed loop (intervention group). Each night was analyzed to the treatment group assigned. Secondary outcomes were calculated for the night (2300 to 0700 h) and 24-h (from midnight to midnight) period. The 24-h analysis used days with evaluable nights and at least 12 h of sensor glucose.
For continuous outcomes, a repeated-measures regression model with an autoregressive first-order covariance structure adjusted for the period effect, adjusted for glucose at 2100 h for night analyses, and based on the ranked normal transformation (except for mean glucose, which was not transformed because it already had an approximate normal distribution) was fit to compare the two treatments. Repeated-measures logistic regression using generalized estimating equations to account for the correlation from the same subject was applied to binary outcomes. Calculations were made using GStat software, version 2.0 (University of Cambridge). Statistical analyses were conducted with the use of SAS software, version 9.3 (SAS Institute, Cary, NC) and SPSS, version 19 (IBM Software, Hampshire, U.K.). Values are reported as mean (SD) or median (interquartile range) unless stated otherwise. All P values are two-tailed, and values <0.05 were considered statistically significant.
Results
We approached 33 and enrolled 17 subjects to attain 16 completed participants. The main reasons for nonparticipation were scheduling logistics (school examinations, family vacations, etc). One enrolled participant did not complete initial training, withdrew consent, and was excluded. Supplementary Fig. 1 shows the flow of participants through the study. Participants included 10 males and 6 females, age 15.6 (2.1) years [mean (SD)]; diabetes duration 7.2 (4.3) years; HbA1c 8.0 (0.9)% [63.9 (9.3) mmol/mol]; BMI 22.4 (3.7) kg/m2; BMI z-score 0.8 (0.8); insulin pump therapy duration of 3.0 (2.3) years; total daily insulin dose 0.8 (0.2) units/kg; and C-peptide–negative (<33 pmol/L), except for three participants with measurable random nonhypoglycemia C-peptide levels of 59, 73, and 394 pmol/L.
Overnight closed loop was used on 311 nights (93%), turned on at 2134 h (2037–2235) and turned off at 0737 h (0701–0909), operating over 10.0 (8.7–11.6) h. On 231 of 311 nights, closed loop did not have any interruption (Supplementary Table 1). Apart from replacement of faulty study devices, the participants were able to resolve most issues on their own, such as restarting closed loop after loss of pump connectivity or sensor data unavailability.
Primary Outcome
Time when adjusted overnight glucose was in the target increased during closed-loop therapy compared with control nights by a median 15% (−9 to 43) from 47% (18–70) to 64% (45–79) (control nights vs. closed-loop nights; P < 0.001). This was corroborated by an increase in unadjusted (raw) overnight sensor glucose by a median 19% (−12 to 50) from 46% (13–77) to 68% (43–86) (P < 0.001) (Supplementary Table 2).
Secondary Efficacy Outcomes Overnight
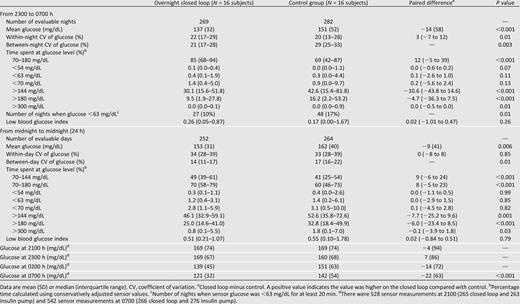
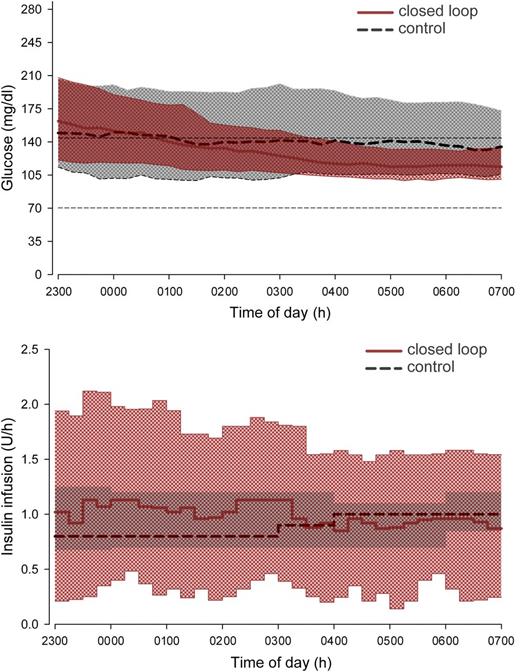
Closed loop reduced overnight glucose by a mean 14 (SD 58) mg/dL, and time above target range was also reduced (both P < 0.001) (Table 1 and Fig. 2). Glucose variability within each night increased by 3% (−7 to 12) (P < 0.01); between-night glucose variability decreased (P < 0.003) with more consistent mean overnight glucose levels (Fig. 3). This was accompanied by consistent morning (0700 h) glucose of 121 (32) mg/dL.
Sensor glucose (top panel) and insulin delivery (bottom panel) from 2300 to 0700 h. Median overnight profiles are represented by red solid (closed loop) and black dashed (control therapy) lines. The interquartile range is shown as red (closed loop) and gray (control therapy) regions. The plots demonstrate progressively tighter overnight glucose levels during closed-loop therapy brought about by more varying insulin delivery, the typical tradeoff of insulin delivery variability for glucose consistency. The target glucose range 70–144 mg/dL is denoted by short dashed lines in the top panel.
Sensor glucose (top panel) and insulin delivery (bottom panel) from 2300 to 0700 h. Median overnight profiles are represented by red solid (closed loop) and black dashed (control therapy) lines. The interquartile range is shown as red (closed loop) and gray (control therapy) regions. The plots demonstrate progressively tighter overnight glucose levels during closed-loop therapy brought about by more varying insulin delivery, the typical tradeoff of insulin delivery variability for glucose consistency. The target glucose range 70–144 mg/dL is denoted by short dashed lines in the top panel.
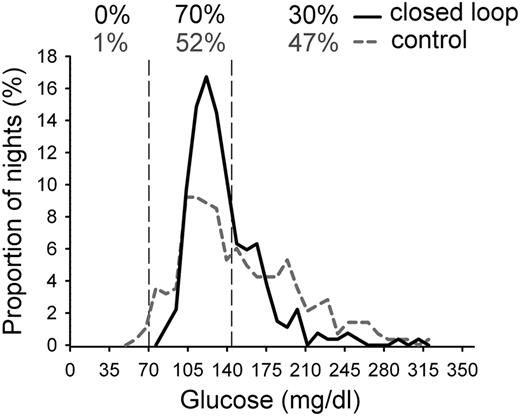
Mean overnight glucose levels during closed-loop and control therapy. Individual mean values were calculated from 2300 to 0700 h. Percentages at the top represent the proportion of nights mean overnight glucose was below, at, and above the target; for example, on 70% of nights, mean overnight glucose was between 70 and 144 mg/dL (vertical dashed lines).
Mean overnight glucose levels during closed-loop and control therapy. Individual mean values were calculated from 2300 to 0700 h. Percentages at the top represent the proportion of nights mean overnight glucose was below, at, and above the target; for example, on 70% of nights, mean overnight glucose was between 70 and 144 mg/dL (vertical dashed lines).
Assessments by unadjusted sensor glucose levels supported the primary end point findings and suggested greater magnitude and wider benefits of closed loop, including reduced time in hypoglycemia and hyperglycemia (Supplementary Table 3). A subanalysis from 0200 to 0700 h demonstrated a more pronounced effect of closed loop after several hours of closed-loop operation (Supplementary Table 6).
Safety and Adverse Events
Time spent in hypoglycemia <70 mg/dL was low in both periods. The number of nights when glucose was <63 mg/dL for at least 20 min was lower during closed loop (10 vs. 17% of nights; P = 0.01). Fingerstick glucose <63 mg/dL further corroborated the reduction in nocturnal hypoglycemia. In total, fingerstick glucose <63 mg/dL was measured on 8 nights during closed loop and 17 control nights.
No serious adverse events were observed during either study period. Two participants measured blood ketones >1.5 mmol/L one participant on one occasion during closed loop and one participant on two occasions during control nights (Supplementary Table 4). These events were attributed to detached infusion sets or incorrect pump priming and were all self-managed.
Secondary Efficacy Outcomes From Midnight to Midnight
Of days included in the night analysis, 12 h of continuous monitoring data were available on 252 (75%) and 264 (79%) study days during closed-loop and control therapy, respectively. Application of overnight closed loop resulted in lower glucose levels until 1100, 3.5 h after stopping closed loop (Supplementary Fig. 3). Twenty-four–hour glucose was reduced by a mean 9 (SD 41) mg/dL (P = 0.006) during closed-loop therapy.
Insulin Requirements
Lower nocturnal glucose levels during closed loop were achieved through increased insulin delivery of 0.9 (−0.5 to 2.8) units in the night period [8.1 (6.5–10.8) vs. 7.2 (5.8–9.1) units; P < 0.001]. However, during the day, 3.2 (−10.2 to 3.4) units less insulin was administered as boluses [25.3 (19.8–33) vs. 28.7 (22–36.4) units; P < 0.001], and the total daily insulin dose decreased by 2.3 (−4.7 to 9.3) units [49.9 (39.6–61.9) vs. 53.2 (42.5–61.7) units; P = 0.009].
Weekly Trends
The number of evaluable nights was similar in each of the 3 weeks (88–91 nights). No weekly trends in glucose control or insulin delivery were observed (Supplementary Table 5).
Conclusions
We have demonstrated the feasibility of extended use of unsupervised overnight closed loop in the home setting. Closed loop increased time spent when glucose was in the target range by a median 15% and reduced both 24-h and overnight glucose levels by a mean 14 and 9 mg/dL, respectively. The number of nights when glucose was <63 mg/dL for at least 20 min was almost halved. Additional benefits included reduced total daily insulin requirements.
After brief operational training (30–60 min), closed-loop technology was simple to initiate, with near-optimal short-term compliance (93% nights) documenting user-friendly interface and simplicity of setup previously assessed during user group sessions. Closed loop was started irrespective of evening glucose levels after performing a safety calibration check. Closed loop increased slightly within-night glucose variability, explained by a drop from evening hyperglycemia to morning euglycemia, whereas sustained elevated glucose was frequent during control nights. Preplanned in-person contacts took place at the start and end of each study period to provide/collect study devices and consumables, take blood samples, and complete questionnaires. Subjects were in weekly telephone/e-mail contact during both study periods. Ad hoc contacts/visits took place to resolve device issues (median 2, range 1–3 in-person visits per participant), mostly occurring during closed-loop intervention.
Closed loop revealed substantial, night-to-night variability in insulin requirements. The amount of insulin delivered by closed loop on individual nights varied between 50 and 200% of the amount given during control nights. During closed loop, the median night-to-night difference in insulin delivery was 27%, with 1 in 10 differences >72%. This explains difficulties in achieving consistent nocturnal glucose levels with conventional and sensor-augmented pump therapy, providing a compelling rationale for the closed-loop approach. The increased variability in insulin delivery during closed loop is offset by reduced variability of glucose concentration, the typical trading of variability associated with closed-loop systems (Fig. 2). The lack of weekly trends confirms both that the benefits of closed loop occurred rapidly and that they can be consistently sustained over multiple nights.
Two short-duration transitional studies assessed the feasibility of a single night of closed loop outside hospital, incorporating real-time telemonitoring and on-site supervision by clinical research staff (20,21). Another home study evaluated overnight closed loop over 4 consecutive nights (22). Prolonged on-site supervision may not be feasible for home studies, and while technological advances may increasingly facilitate remote telemonitoring approaches, it limits implementation in real-life clinical settings. The only adverse events observed in our study were three instances of elevated ketones, most likely related to unfamiliarity with the study pump and infusion set failures. They were all successfully self-managed, suggesting that additional intensive supervision is not required for safe application of closed loop.
The amount of nocturnal hypoglycemia was less than half that compared with the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Trial in youth (30). Despite low baseline levels, the number of nights when sensor glucose was <63 mg/dL for ≥20 min was reduced. Phillip et al. (20) also showed that closed loop reduced the number of nights with nocturnal hypoglycemia within a diabetes camp setting, but with higher baseline prevalence and higher mean glucose compared with our results. Nimri et al. (22) in an interim analysis showed reduction of hypoglycemia duration during 4-night home use of closed loop without change in mean glucose.
To avoid overestimating the potential impact of closed loop, we performed all statistical analyses using both raw and adjusted sensor data confirming a 19 and 15% increased time in target, respectively. Potential limitations include a small number of participants, which may limit generalizability, and a relatively short duration. However, participants’ characteristics are comparable to the U.S. T1D Exchange Registry (3) and U.K. national audit (31), demonstrating that we studied a representative population of youth with type 1 diabetes. The strength of the adolescents' overnight closed-loop study is the integration of closed loop into a normal living routine including school/weekdays, weekends, holidays, and with varied diet and sleeping patterns. Without supervision, adolescents with type 1 diabetes started and stopped closed loop by their own volition over multiple nights and without real-time monitoring or redundant sensor, which will accelerate cost-effective transition into routine clinical care.
In conclusion, overnight closed loop was safe, and its benefits included increased time when glucose is in target, reduced mean glucose, and fewer nights with hypoglycemia. Further longer-term studies are warranted.
A slide set summarizing this article is available online.
Clinical trial reg. no. NCT01221467, clinicaltrials.gov.
Article Information
Acknowledgments. The authors thank the study volunteers for participation, Prof. Peter Hindmarsh (University College, London, U.K.) for help in identifying potential recruits, John Lum (Jaeb Centre) and Jasdip Mangat (Cambridge University Hospitals NHS Foundation Trust) for supporting development and validation of the closed-loop system, Josephine Hayes (Institute of Metabolic Science, University of Cambridge) for administrative support, Arti Gulati (University of Cambridge) for providing data management support, Karen Whitehead (University of Cambridge) for providing laboratory support, and The Core Biochemical Assay Laboratory, University of Cambridge (Keith Burling) for carrying out biochemical analyses.
Funding. Supported by the Juvenile Diabetes Research Foundation (grants 22-2006-1113, 22-2007-1801, 22-2009-801, and 22-2009-802), Diabetes UK (BDA07/0003549), the National Institute of Diabetes and Digestive and Kidney Diseases (1R01-DK-085621), the Medical Research Council Centre for Obesity and Related Metabolic Diseases, and National Institute for Health Research Cambridge Biomedical Research Centre. Abbott Diabetes Care supplied continuous glucose delivery devices, sensors, and modified devices to facilitate real-time connectivity. Abbott Diabetes Care read the manuscript before submission. No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Duality of Interest. R.H. received speaker honoraria from Medtronic, LifeScan, Eli Lilly, B. Braun Medical Inc., and Novo Nordisk; served on an advisory panel for Animas, Medtronic, and Eli Lilly; received license fees from B. Braun Medical Inc. and BD Biosciences; and served as a consultant to BD Biosciences, B. Braun Medical Inc., Sanofi, and Profil. C.K. served as a consultant to Medtronic International Trading Sàrl and Diabetes Technology Management. H.R.M. received speaker honoraria from MiniMed Medtronic. M.E.W. received license fees from and has served as a consultant to BD Biosciences. R.H., M.E.W., and D.B.D. report patent applications. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.H. coordinated the study, co-designed the studies, designed and implemented the glucose controller, and contributed to the interpretation of the results. D.E. co-designed the studies, was responsible for screening and enrollment of participants and arranged informed consent from the participants, provided patient care and/or took samples, carried out or supported data analysis, including the statistical analyses, and contributed to the interpretation of the results. H.T., L.L., K.K., and K.C. provided patient care and/or took samples. J.M.A. co-designed the studies, was responsible for screening and enrollment of participants and arranged informed consent from the participants, and provided patient care and/or took samples. R.E.-K. was responsible for screening and enrollment of participants and arranged informed consent from the participants, provided patient care and/or took samples, and contributed to the interpretation of the results. P.C. and C.K. carried out or supported data analysis, including the statistical analyses. H.R.M. and C.L.A. co-designed the studies and contributed to the interpretation of the results. M.E.W. co-designed the studies and carried out randomization. M.N. carried out or supported data analysis, including the statistical analyses, and contributed to the interpretation of the results. D.B.D. co-designed the studies and contributed to the interpretation of the results. All authors critically reviewed the report. R.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.