Weight loss reduces abdominal and intrahepatic fat, thereby improving metabolic and cardiovascular risk. Yet, many patients regain weight after successful diet-induced weight loss. Long-term changes in abdominal and liver fat, along with liver test results and insulin resistance, are not known.
We analyzed 50 overweight to obese subjects (46 ± 9 years of age; BMI, 32.5 ± 3.3 kg/m2; women, 77%) who had participated in a 6-month hypocaloric diet and were randomized to either reduced carbohydrates or reduced fat content. Before, directly after diet, and at an average of 24 (range, 17–36) months follow-up, we assessed body fat distribution by magnetic resonance imaging and markers of liver function and insulin resistance.
Body weight decreased with diet but had increased again at follow-up. Subjects also partially regained abdominal subcutaneous and visceral adipose tissue. In contrast, intrahepatic fat decreased with diet and remained reduced at follow-up (7.8 ± 9.8% [baseline], 4.5 ± 5.9% [6 months], and 4.7 ± 5.9% [follow-up]). Similar patterns were observed for markers of liver function, whole-body insulin sensitivity, and hepatic insulin resistance. Changes in intrahepatic fat und intrahepatic function were independent of macronutrient composition during intervention and were most effective in subjects with nonalcoholic fatty liver disease at baseline.
A 6-month hypocaloric diet induced improvements in hepatic fat, liver test results, and insulin resistance despite regaining of weight up to 2 years after the active intervention. Body weight and adiposity measurements may underestimate beneficial long-term effects of dietary interventions.
Increases in visceral and subcutaneous abdominal fat as well as ectopic fat deposition contribute to the development of metabolic abnormalities in obesity (1). In particular, intrahepatic fat accumulation is associated with increased insulin resistance and promotes the development of type 2 diabetes (2,3), independently of total or visceral fat mass (4,5). Excessive hepatic fat also predisposes to nonalcoholic steatohepatitis, which may progress to cirrhosis and hepatic cancer (6). Thus, interventions reducing hepatic fat address the root cause for both obesity-associated metabolic disease and liver disease. Lifestyle interventions including hypocaloric diets are a cornerstone of obesity management because diet-induced weight loss improves insulin sensitivity (7) while preventing type 2 diabetes (8). Weight reduction through caloric restriction decreased hepatic fat in studies lasting up to 12 months (9,10). The improvement in hepatic fat during dieting was primarily related to caloric restriction rather than macronutrient composition (11). Two important issues are involved in weight reduction studies. First, there may be dissociation between body weight changes and cardiovascular and metabolic risk factors over time. For example, whereas bariatric surgery decreases the risk for new-onset diabetes for many years, the risk for arterial hypertension may not be reduced despite sustained weight loss (12). Second, many subjects regain weight after diet-induced weight loss (13). Whether weight regain negates previous improvements in hepatic fat and liver function has not been investigated. Given the importance of hepatic fat in the pathogenesis of obesity-associated metabolic disease, we assessed long-term changes in visceral fat, subcutaneous fat, liver fat, liver test results, and insulin resistance after dietary weight loss in overweight or obese subjects.
RESEARCH DESIGN AND METHODS
Participants
Between March 2007 and June 2010, 108 overweight and obese subjects without signs and symptoms of cardiac, vascular, renal, metabolic, and gastrointestinal diseases completed a 6-month hypocaloric diet (−30% energy intake) with either reduced carbohydrate or reduced fat content. Subject characteristics had been described previously (11). Subjects received no medications and required no other medical care. We excluded subjects reporting more than 2 h of physical activity per week, subjects consuming >20 g/day of alcohol, and pregnant or nursing women. All subjects who completed the study were invited to participate in a long-term follow-up. The study was performed in accordance with the Declaration of Helsinki (1996). Our Institutional Review Board approved the study and written informed consent was obtained before entry.
Study design
The follow-up investigation presented here is part of the B-SMART study (Clinical trial reg. no. NCT00956566, clinicaltrials.gov), which compared weight loss and changes of associated metabolic and cardiovascular markers in hypocaloric diets with reduced carbohydrates and reduced fat. The randomized 6-month dietary intervention consisted of individual and group dietary counseling with the goals of reducing energy intake by 30% with a minimum of 1,200 kcal/day and adherence to one diet or the other. The reduced carbohydrate diet contained ≤90 g carbohydrates, 0.8 g protein/kg body weight, and ≥30% fat. The reduced-fat diet contained ≤20% fat, 0.8 g protein/kg body weight, and the remaining energy content comprised carbohydrates. All subjects who completed the 6-month diet were invited to participate in a follow-up visit. The only contacts during follow-up were occasional telephone calls to monitor weight changes and to invite subjects to the final examination. No intervention of any kind was offered during follow-up. The follow-up visit occurred between 17 and 36 months after completion of the supervised dietary intervention (Supplementary Fig. 1). All anthropometric, metabolic, and magnetic resonance imaging studies were conducted in an academic clinical research center with the same equipment, scientific staff, and protocols as applied during the baseline and postdiet measurements (11).
Anthropometric and metabolic evaluation
We measured body weight, waist circumference, and height in a standardized manner after an overnight fast. We obtained blood samples at baseline and at 15, 30, 45, 60, 90, and 120 min after glucose ingestion (75 g glucose/300 mL; oral glucose tolerance test [OGTT]) to measure glucose and insulin. Imaging studies were performed after another overnight fast. Participants provided a 7-day food protocol that was analyzed for energy intake and macronutrient content using Optidiet (V3.1.0.004; GOE, Linden, Germany), an analysis software based on nutritional content of food as provided by the German National Food Key.
Abdominal and liver fat imaging
A clinical 1.5-T magnetic resonance scanner (Sonata and Avanto; Siemens Medical Solution AG, Erlangen, Germany) was used to measure abdominal subcutaneous and visceral fat mass as well as liver fat content as previously described (14). Briefly, we applied a T1-weighted, water-suppressed, gradient echo technique (repetition time, 80 ms; echo time, 6.11 ms; 512 × 512 matrix; field of view, 500 × 500 mm; slice thickness, 10 mm; interslice gap, 10 mm) to image abdominal fat during repetitive breath-holds. Axial slices were acquired from the diaphragm to symphysis. We quantified visceral and subcutaneous adipose tissue by semi-automated image segmentation software using a contour-following algorithm (Vitom). In addition, we measured intrahepatic lipids by respiratory-gated 1H spectrometry (spin-echo: repetition time according to respiratory cycle (>5 s); echo time, 30 ms). Unsuppressed spectra were acquired in end-expiration from a single 30- × 30- × 20-mm3 voxel located at liver segment 7 (24 averages). Intrahepatic lipid content was quantified using peak areas and expressed (as percent) as fat ÷ (fat + water).
Biochemical measurements and calculations
Glucose (mmol/L), insulin (µU/mL), lipoproteins, alanine aminotransferase (U/L), aspartate aminotransferase (U/L), and γ-glutamyltransferase (U/L) were determined by standard methods in a certified clinical chemistry laboratory. Insulin resistance was estimated by homeostasis model assessment of insulin resistance index. Homeostasis model assessment of insulin resistance was calculated from fasting insulin and glucose by the following equation: (insulin [µU/mL] × glucose [mmol/L]) ÷ 22.5). Whole-body insulin sensitivity was calculated by the composite insulin sensitivity index (15). Composite insulin sensitivity index = 10,000 ÷ √[(fasting plasma glucose × fasting plasma insulin) × (glucose × insulin)]; fasting plasma glucose was expressed as mg/dL and fasting plasma insulin was expressed as µU/mL, and glucose (mg/dL) and insulin (µU/mL) were the mean glucose and mean insulin concentrations during the glucose load. The hepatic insulin resistance index was estimated from the results of the OGTT. This approach has been validated in nondiabetic subjects by using euglycemic insulin clamp testing including labeled glucose administration (16).
Statistical analysis
Data were first tested for normal distribution and variance homogeneity with Kolmogorov-Smirnov test and the Levene test, respectively. Differences between time points (baseline, after diet, follow-up) were analyzed using ANOVA for repeated measures with Bonferroni post hoc test. Univariate associations between parameters were described by Pearson correlation coefficient. To test for interactions between diet groups or weight loss groups over time, we used two-way ANOVA for repeated measures and Bonferroni post hoc test. To identify independent predictors of hepatic fat content at baseline and of long-term reduction in hepatic fat after the dietary intervention, we conducted multivariate linear regression analyses. All statistical analyses were performed with SPSS 18 (SPSS, Chicago, IL). Significance was accepted at P < 0.05. Unless otherwise stated, values are given as mean ± SD. Post hoc power analysis was calculated with G*Power 3.1.7 (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3).
RESULTS
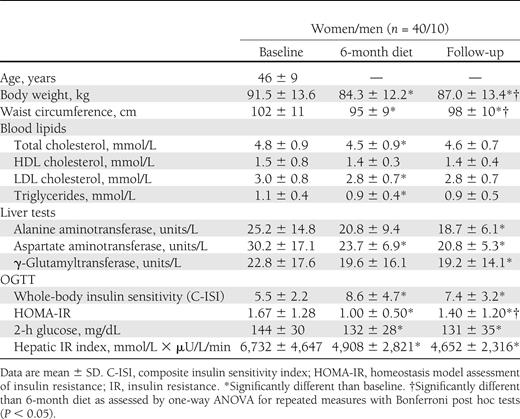
Fifty subjects had complete data sets at follow-up and were included in the analysis (Table 1). Participant flow is shown in Supplementary Fig. 1. The time between completion of diet and follow-up visit was 24 ± 6 months (range, 17–36). The long noninterventional follow-up period was associated with a substantial discontinuation rate (54 of 108 eligible subjects participated in the follow-up visit). Approximately half of the subjects changed housing and contact details without notifying the investigators (lost to contact). The other subjects withdrew consent to further participation because of time constraints or disinterest in further scientific evaluations. To test the possibility that the high discontinuation rate introduced a substantial bias, we compared participants and nonparticipants with respect to baseline data and changes at 1, 3, and 6 months after diet initiation (Supplementary Table 1). Subjects who discontinued the follow-up period had similar age, baseline BMI, and improvements in anthropometric and metabolic parameters during the active intervention period compared with those who participated in the follow-up evaluation.
Anthropometric and metabolic variables after the 6-month diet intervention and at follow-up 17–36 months after diet
Total energy intake was reduced during the dietary intervention period (P < 0.01) and remained reduced at follow-up (baseline: 2,245 ± 619 kcal/d; end of diet: 1,720 ± 450 kcal/d; follow-up: 1,836 ± 468 kcal/d). To analyze predictors of baseline hepatic fat content, we conducted a multivariate regression analysis with age, sex, BMI, insulin sensitivity, total cholesterol, LDLs, HDLs, triglycerides, free fatty acids, visceral and subcutaneous abdominal fat mass, cardiorespiratory fitness (Vo2max), total energy intake, and percentage of fat and carbohydrate intake relative to total energy intake. The analysis revealed only visceral fat mass (β = 0.64; P = 0.006) as an independent predictor of baseline hepatic fat content.
We calculated weight regain as the difference between body weight at follow-up and body weight after the active dietary intervention period at 6 months. After successful reduction of body weight and BMI with dietary intervention, we observed a significant weight regain at follow-up (Table 1 and Fig. 1). The concomitant increase in visceral and subcutaneous abdominal fat mass followed a parallel trend (Fig. 1). In contrast, intrahepatic lipids decreased during the dietary intervention but remained reduced at follow-up (Fig. 1). Body weight regain was correlated with a regain in visceral (r = 0.70; P < 0.001) or subcutaneous abdominal adipose tissue (r = 0.90; P < 0.001), but less so with increased hepatic fat content (r = 0.30; P < 0.05). Long-term reduction of hepatic lipids was associated with sustained improvements in serum alanine aminotransferase, aspartate aminotransferase, and γ-glutamyltransferase activities (Table 1). Finally, indices of insulin resistance were obtained from OGTT. All measured data and calculated variables improved with the active diet and remained so at follow-up (Table 1 and Fig. 2). When analyzed separately for subjects initially randomized to reduced-carbohydrate or reduced-fat diets, all analyzed variables changed similarly in both groups over time.
BMI, abdominal fat, and intrahepatic fat after 6-month diet and at follow-up. Changes in BMI and subcutaneous abdominal adipose tissue (AT) (upper panel) and visceral AT and intrahepatic lipids (lower panel) in 50 overweight and obese subjects. Magnetic resonance studies were performed at baseline, after a 6-month hypocaloric diet, and at long-term follow-up. *P < 0.01 compared with baseline. †P < 0.01 compared with end of diet. Group comparison by one-way ANOVA for repeated measures with Bonferroni post hoc test. Data are mean ± SEM.
BMI, abdominal fat, and intrahepatic fat after 6-month diet and at follow-up. Changes in BMI and subcutaneous abdominal adipose tissue (AT) (upper panel) and visceral AT and intrahepatic lipids (lower panel) in 50 overweight and obese subjects. Magnetic resonance studies were performed at baseline, after a 6-month hypocaloric diet, and at long-term follow-up. *P < 0.01 compared with baseline. †P < 0.01 compared with end of diet. Group comparison by one-way ANOVA for repeated measures with Bonferroni post hoc test. Data are mean ± SEM.
Glucose and insulin concentrations during OGTT after 6-month diet and at follow-up. Time course and areas under the curve (AUC) for glucose (upper panel) and insulin (lower panel) during a 2-h OGTT (n = 46). The test was performed at baseline, after a 6-month hypocaloric diet, and at long-term follow-up. *P < 0.01 compared with baseline. Group comparison by one-way ANOVA for repeated measures and Bonferroni post hoc test. Data are mean ± SEM.
Glucose and insulin concentrations during OGTT after 6-month diet and at follow-up. Time course and areas under the curve (AUC) for glucose (upper panel) and insulin (lower panel) during a 2-h OGTT (n = 46). The test was performed at baseline, after a 6-month hypocaloric diet, and at long-term follow-up. *P < 0.01 compared with baseline. Group comparison by one-way ANOVA for repeated measures and Bonferroni post hoc test. Data are mean ± SEM.
Hepatic fat reduction is particularly relevant in subjects with nonalcoholic fatty liver disease (NAFLD). We stratified subjects into a group with NAFLD and a group without NAFLD at baseline (hepatic fat >5.6% or <5.6%; Table 2). Groups were similar in age, body weight, and sex distribution. Both groups lost similar amounts of body weight and visceral and subcutaneous abdominal fat mass with the same total energy intake reduction during dietary intervention. Subjects with NAFLD showed sustained improvement in hepatic fat content and liver function despite modest weight regain during follow-up.
Changes with 6-month diet and at long-term follow-up in subjects stratified for the presence of NAFLD at baseline

We conducted a multivariate regression analysis using age, sex, baseline BMI, type of diet, changes in body weight with diet, changes in visceral and subcutaneous fat mass with diet, changes in hepatic fat with diet, changes in total cholesterol with diet, and changes in insulin sensitivity with diet as independent variables; changes from baseline to long-term follow-up of liver fat was used as the dependent variable. Only changes in total body weight with diet (β = 0.31; P = 0.02) and changes in hepatic fat with diet (β = 0.86; P < 0.001) predicted long-term intrahepatic fat loss. The model explained 68% of the total variation in the observed long-term liver fat reduction.
CONCLUSIONS
The important and novel finding of our study is a sustained improvement in hepatic fat content, liver function tests, and insulin resistance over >2 years after a 6-month hypocaloric diet in overweight and obese subjects. All improvements occurred despite regain of body weight, abdominal visceral adipose tissue, and subcutaneous adipose tissue mass during follow-up. Our findings highlight the beneficial long-term effects of a well-controlled dietary lifestyle intervention on hepatic fat content and metabolism. Furthermore, our findings suggest that the benefit of dietary interventions should not be judged solely by anthropometric measurements. In fact, reductions in caloric intake may have weight-independent effects on liver fat and metabolism.
Various types of caloric restriction are effective in decreasing body weight (13) and fat mass (17). Whereas short-term reductions of body weight and fat mass can be impressive, most studies with long-term follow-up were discouraging and reported at least partial body weight regain within 1–2 years (13). Data regarding changes of visceral adipose tissue and ectopic fat storage during long-term follow-up are scarce. The issue is relevant because visceral adipose tissue is independently associated with cardiovascular and metabolic risk (1). Furthermore, ectopic fat storage in liver, skeletal muscle, pancreas, and the heart adversely affects organ function through a mechanism often referred to as “lipotoxicity.” Intrahepatic fat content is an important predictor of whole-body insulin resistance, increased secretion rates of VLDLs, and progression to type 2 diabetes (2,4,18). Type 2 diabetes, in turn, increases the risk of progressive liver disease (e.g., nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma) (19–21). Our study is the first to assess abdominal fat mass and liver fat several months after diet-induced weight loss and has scientific as well as clinical implications.
Abdominal visceral adipose tissue drains into the portal vein and the liver is exposed to large amounts of free fatty acids derived from this metabolically active fat depot. In our study, hepatic fat at baseline was strongly related to visceral fat mass. Yet, visceral adipose tissue mass may not be the sole determinant of how hepatic fat responds to dietary interventions (22). Short-term studies suggested that hepatic fat accumulation is, at least in part, regulated independently of body weight and abdominal visceral fat mass. In obese subjects, caloric restriction decreased hepatic fat by 10–30% within 48 h (23). Furthermore, <5% weight loss decreased hepatic fat by 28–40% (24–26). Finally, aerobic exercise training ameliorated hepatic fat content while body weight (27) and visceral fat mass (28) remained stable. Our study extends these observations and suggests that differential regulation of adipose tissue mass and hepatic fat stores may not be restricted to the acute weight loss period. Moreover, our observations further support the idea that intrahepatic fat is independently associated with metabolic risk (4,5,29).
There is an ongoing debate whether hepatic insulin resistance is primarily mediated through fatty acid and adipokine release from expanded and dysfunctional visceral adipose tissue or through generation of intrahepatic lipid mediators, such as diacylglycerols and ceramides that directly interfere with insulin signaling (30). Our finding that insulin sensitivity remained improved during follow-up despite the weight and fat mass regain strengthens the notion that intrahepatic lipids and their metabolites are mediators of hepatic insulin resistance.
We observed a constantly reduced energy intake during follow-up but regain of body weight and fat mass. A possible explanation is that weight loss and caloric restriction led to compensatory reductions in metabolic rate (31). Approximately 80% of liver fatty acids originate from circulating fatty acids (32) that are mobilized predominantly from adipose tissue. Improved insulin resistance with weight loss attenuates adipose tissue lipolysis and enhances fatty acid storage within the large adipose tissue depots. Fatty acids are taken-up into liver and adipose tissue through fatty acid transport proteins and FAT/CD36 (33). In obese subjects, intrahepatic lipid content determined the expression of fatty acid transporters and FAT/CD36 in liver and adipose tissue. Fatty acid transporters were expressed to a lesser extent in the liver and to a higher extent in adipose tissue when subjects had normal liver fat content, and this was reversed in subjects with high liver fat content, suggesting that fatty acid flux from adipose tissue to the liver determines liver fat content (33). Overall, our study suggests that successful weight loss achieved through hypocaloric dieting improves fatty acid flux from the liver toward the primary storage site in the long-term. The same pattern, rerouting of free fatty acids from the liver toward adipose tissue together with improved insulin sensitivity, also has been observed with thiazolidinedione treatment (34).
Our clinically important observation is that a controlled 6-month intervention elicited sustained lifestyle changes in a surprisingly large proportion of our subjects. Caloric intake was still reduced during the follow-up assessment. Thus, important lifestyle improvements may be obscured if the focus is on weight changes only. We also observed that similar sustained improvements in hepatic fat, markers of liver function, and insulin resistance occurred in subjects initially assigned to carbohydrate-reduced or fat-reduced diets. This finding extends our results observed at the end of the 6-month diet period (11) and suggests that changes in energy balance may be more important than changes in dietary composition for sustained hepatic fat reduction. Also clinically important is the observation that subjects fulfilling diagnostic criteria of NAFLD had a particularly pronounced reduction in hepatic fat over time. NAFLD affects up to 30% of adults in developed countries (35) and is related to the ongoing obesity epidemic (3). The condition is an independent risk factor for cardiovascular disease and type 2 diabetes, predisposes to nonalcoholic steatohepatitis, and may progress to cirrhosis and hepatic cancer (6). The 45% reduction of hepatic fat with hypocaloric diets observed in this study is a particular benefit in subjects with NAFLD. Our findings suggest that this important improvement in hepatic liver fat can be maintained in the long-term even when body weight is partly regained.
The major limitation of our study is that 50% of the subjects who had finished the active intervention period discontinued participation during the follow-up period. Discontinuation rates ranged between 33 and 50% in two recent trials reporting 1-year treatment data for Food and Drug Administration–approved weight loss drugs (36,37). In these studies, discontinuation occurred during the active treatment period, whereas our participants discontinued during an observational period after the active intervention while contact was maintained through occasional telephone calls only. Clearly, more close contact may have increased the retention rate; however, the long-term value of our weight loss program for the influence on hepatic steatosis could not have been assessed as planned because close and regular contact represents an intervention by itself. Instead, we wanted to determine the impact of the intervention with the least interaction with subjects as possible. This study design, however, resulted in the problem of losing contact with approximately half of the subjects who discontinued participation. The other half discontinued by withdrawing consent; however, if we were informed correctly by these subjects, withdrawn consent was not related to unsuccessful weight loss or any other undesired developments during follow-up. Based on mathematical models estimating discontinuation rates in dietary intervention studies (38,39), the rate in our study is in the expected range. However, subjects more successful at losing weight and maintaining their diets could be more motivated to participate in follow-up investigations. As shown in Supplementary Table 1, subjects who discontinued participation did not differ from participants included in the follow-up analysis in terms of baseline anthropometric measurements, metabolism, or early and sustained weight loss success during the intervention period. Furthermore, we observed no differences in sex distribution between participants and those who discontinued study participation. The analysis suggests that discontinuation was not limited to a specific patient population, thus rendering a major bias less likely. To test whether the sample size was sufficiently large to assess the reduction in intrahepatic fat from baseline to 24-month follow-up, we conducted a post hoc power calculation. With a statistical power of 80%, a sample size of 33 would have been sufficient to detect the observed difference in liver fat of 3.1 ± 6.1% with a one-sided α-error of 0.025. Given the inclusion of 50 subjects in our study, the probability of the detected difference with a one-sided α-error of 0.025 was 94%. Nevertheless, losing 50% of participants could have introduced bias; therefore, results should be interpreted with caution.
There are other methodological limitations of our study. First, we did not perform liver biopsies. Thus, we cannot prove that the sustained improvement in hepatic fat in our study was associated with beneficial changes in hepatic inflammation and fibrosis. In contrast to steatosis, advanced liver fibrosis is less amenable to therapeutic interventions (35). Second, we are aware that hyperinsulinemic euglycemic clamping would have provided more direct information regarding insulin sensitivity compared with OGTT. Third, we did not measure food intake directly. Because of the possibility of underreporting, the magnitude of the reduced caloric intake after diet and at follow-up needs to be interpreted with caution (40).
Despite these limitations, particularly the high discontinuation rate, we conclude that hypocaloric dietary interventions have a long-lasting effect on intrahepatic lipid accumulation despite weight regain. Because obesity management programs commonly focus on body weight and anthropometric measurements, beneficial long-term effects of dietary interventions on human metabolism may be underestimated. We suggest that the beneficial response to reduced caloric intake in our study may be driven in large part by changes in liver metabolism.
Clinical trial reg. no. NCT00956566, clinicaltrials.gov.
Acknowledgments
This study was part of a joint project between Metanomics GmbH (Berlin, Germany) and Charité University Medical School, which was supported by the Federal Ministry of Education and Research (BMBF-0313868). A.L.B. was supported by a grant from the German Research Foundation (BI1292/4-1). The German Competence Network of Obesity (projects 01Gl0830 and 01GI1122D; S.E.) supported the project.
No potential conflicts of interest relevant to this article were reported.
S.H. wrote the manuscript and researched data. V.H. and W.U. researched data and contributed to discussion. A.L.B. and J.S.-M. contributed to discussion and reviewed and edited the manuscript. S.J. researched data. J.B. conceived and designed the study and researched data. A.M. researched data. F.C.L. and J.J. conceived and designed the study, contributed to discussion, and reviewed and edited the manuscript. M.B. conceived and designed the study and contributed to discussion. S.E. conceived and designed the study, researched data, and wrote the manuscript. All authors had access to the study data and reviewed and approved the final manuscript. S.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Gritt Stoffels, Anke Strauss, and Elke Nickel-Sczcech (all affiliated with Franz Volhard Clinical Research Center at the Experimental and Clinical Research Center, Charité University Medical School, and Max Delbrück Center for Molecular Medicine, Berlin, Germany) for expert technical help.






