Insulin recruits muscle microvasculature, thereby increasing endothelial exchange surface area. Free fatty acids (FFAs) cause insulin resistance by activating inhibitor of κB kinase β. Elevating plasma FFAs impairs insulin’s microvascular and metabolic actions in vivo. Whether salsalate, an anti-inflammatory agent, prevents FFA-induced microvascular and/or metabolic insulin resistance in humans is unknown.
Eleven healthy, young adults were studied three times in random order. After an overnight fast, on two occasions each subject received a 5-h systemic infusion of Intralipid ± salsalate pretreatment (50 mg/kg/day for 4 days). On the third occasion, saline replaced Intralipid. A 1 mU/kg/min euglycemic insulin clamp was superimposed over the last 2-h of each study. Skeletal and cardiac muscle microvascular blood volume (MBV), microvascular flow velocity (MFV), and microvascular blood flow (MBF) were determined before and after insulin infusion. Whole body glucose disposal rates were calculated from glucose infusion rates.
Insulin significantly increased skeletal and cardiac muscle MBV and MBF without affecting MFV. Lipid infusion abolished insulin-mediated microvascular recruitment in both skeletal and cardiac muscle and lowered insulin-stimulated whole body glucose disposal (P < 0.001). Salsalate treatment rescued insulin’s actions to recruit muscle microvasculature and improved insulin-stimulated whole body glucose disposal in the presence of high plasma FFAs.
High plasma concentrations of FFAs cause both microvascular and metabolic insulin resistance, which can be prevented or attenuated by salsalate treatment. Our data suggest that treatments aimed at inhibition of inflammatory response might help alleviate vascular insulin resistance and improve metabolic control in patients with diabetes.
Insulin resistance and vascular dysfunction play key roles in the development of cardiovascular complications in patients with type 2 diabetes. Among many contributing factors, free fatty acids (FFAs) have been implicated as the key culprit. As major oxidative fuels for heart and resting muscle, plasma levels of FFAs are elevated in patients with diabetes and they induce insulin resistance, inflammation, and vascular dysfunction.
In cultured endothelial cells (ECs), the inhibitor of κB kinase β (IKKβ) appears to be the common effector in FFA-mediated vascular insulin resistance and inflammation, as transfection of the ECs with a dominant negative IKKβ abrogates FFA-mediated insulin resistance, whereas overexpression of wild-type IKKβ recapitulates the effect of FFAs (1). IKKβ is a serine kinase that controls the activation of nuclear factor-κB and is a critical link between inflammation and insulin resistance (2–4). To date, evidence strongly suggests that FFAs induce inflammation, which contributes to the pathogenesis of insulin resistance and endothelial dysfunction in diabetes.
We have previously shown that insulin at physiological concentrations potently increases microvascular recruitment in both cardiac (5,6) and skeletal muscle (6,7) in humans. Insulin’s vasodilatory effect appears to be mediated through the phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B/endothelial nitric oxide (NO) synthase (eNOS) pathway (8). This insulin-mediated microvascular recruitment is impaired in the presence of high plasma concentrations of FFAs (6,7,9). Whether inflammation is involved in this FFA-induced microvascular insulin resistance is not clear.
In the current study, we examined the effect of salsalate, an anti-inflammatory agent that inhibits IKKβ, on lipid-induced microvascular and metabolic insulin resistance in healthy humans. Our results suggest that lipid likely induces microvascular and metabolic insulin resistance in humans via an inflammation-mediated mechanism, as salsalate treatment restored muscle microvascular insulin response, which was associated with decreased metabolic insulin resistance in the presence of high plasma concentrations of FFAs.
RESEARCH DESIGN AND METHODS
Study protocols
Young, healthy subjects with no history of obesity, hypertension, diabetes, or hyperlipidemia were screened in the General Clinical Research Center (GCRC) at the University of Virginia. Prothrombin time, partial prothrombin time, complete blood counts, lipid profiles, insulin, and glucose were measured. Height and weight were measured and the BMI was calculated. Exclusion criteria included pregnancy, smoking, anemia, bleeding tendency, taking medications or supplements that are known to affect endothelial function and/or glucose metabolism, and family history of diabetes in a first-degree relative.
A total of 11 (8 men, 3 women) subjects (age 21.7 ± 0.4 years, BMI 23.2 ± 0.7 kg/m2) were recruited and studied three times in the GCRC. All subjects provided written informed consent at the screening visit, and the study protocol was approved by the University of Virginia Institutional Review Board and the GCRC Advisory Committee.
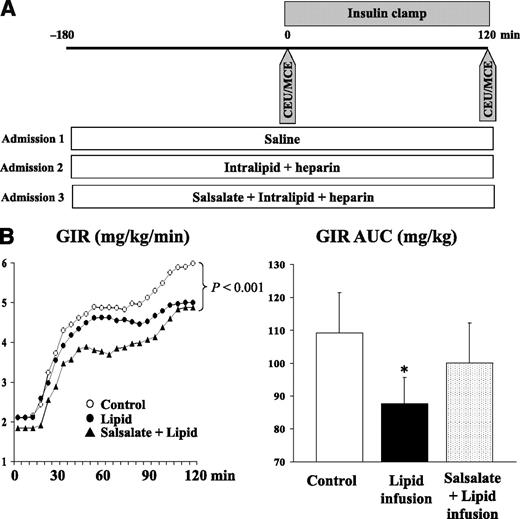
At each study, subjects were admitted the evening before and fasted overnight. On the morning of the study, one intravenous catheter was placed in the right antecubital vein for the infusion of normal saline, Intralipid with heparin, microbubbles, insulin, and glucose. A second intravenous catheter was placed in the right hand for blood sampling of glucose, FFAs, and insulin. Subjects were studied under the following three protocols in random order, with at least 2 weeks between admissions (Fig. 1A).
Study protocol (A) and GIRs during euglycemic-hyperinsulinemic clamps (B). Each subject was studied thrice randomly and CEU/MCE performed before and after 2-h euglycemic insulin clamp. Plasma glucose was measured every 5 min and GIR was adjusted to maintain euglycemia. AUC, area under the curve. *P < 0.007 vs. control.
Study protocol (A) and GIRs during euglycemic-hyperinsulinemic clamps (B). Each subject was studied thrice randomly and CEU/MCE performed before and after 2-h euglycemic insulin clamp. Plasma glucose was measured every 5 min and GIR was adjusted to maintain euglycemia. AUC, area under the curve. *P < 0.007 vs. control.
Saline (control) protocol.
After obtaining baseline blood samples, baseline skeletal and cardiac muscle microvascular parameter measurements—including microvascular blood volume (MBV) and microvascular flow velocity (MFV)—were determined using contrast-enhanced ultrasound (CEU) and myocardial contrast echocardiography (MCE), and microvascular blood flow (MBF) was calculated (see below). Each subject then received a 2-h intravenous infusion of regular insulin (2 mU/min/kg × 10 min and 1 mU/min/kg × 110 min). Plasma glucose was measured every 5 min throughout the insulin infusion, and 20% dextrose was infused at variable rates to maintain euglycemia. Skeletal and cardiac muscle MBV and MFV were again measured, and MBF was calculated at the end of the 120 min insulin clamp. Plasma insulin and FFAs were measured at 0, 30, 60, 90, and 120 min.
Lipid protocol.
The lipid study was carried out in the exact same fashion as the saline control protocol except that all subjects received intravenous infusion of Intralipid (20%, 45 mL/h) and heparin (0.2 units/kg/min) for a total of 5 h. Heparin was given to activate endothelial lipoprotein lipase to facilitate the conversion of circulating lipids to FFAs. The insulin clamp was started 3 h after the initiation of the Intralipid/heparin infusion and the CEU/MCE measurements were done immediately before and at 120 min of insulin infusion.
Salsalate plus lipid protocol.
The salsalate plus lipid study was identical to the lipid protocol except that all subjects received salsalate 25 mg/kg twice a day by mouth for 4 days prior to study. The last salsalate dose was given at time −180 min on the day of study (Fig. 1A). Plasma salicylate concentrations were 22 ± 1 mg/dL at time −180 min (prior to the last dose) and 25 ± 2 mg/dL at time 0 min (3 h after the last dose), suggesting that the plasma salsalate concentrations had reached a relative steady state. This dose was well tolerated by all subjects.
Imaging procedure and analyses
CEU and MCE were performed using a SONOS 7500 ultrasound system and an S3 phased array transducer (Philips Medical Systems, Andover, MA) while the subject was in the left decubitus position, as previously described (5,7). In brief, the contrast agent (Definity, Lantheus Medical Imaging, North Billerica, MA) was infused intravenously, and after the systemic microbubble concentrations reached steady state (∼3 min), intermittent imaging of the forearm was performed in a transaxial plane 5 cm distal to the antecubital fossa using ultraharmonic imaging with pulsing intervals (PIs) ranging from 1 to 20 s. Immediately after the forearm muscle CEU imaging, myocardial microvascular imaging was done using intermittent ultraharmonic imaging from the apical 4-, 2-, and 3-chamber windows, with ultrasound transmitted at 1.3 MHz and received at 3.6 MHz and PIs of 1, 2, 3, 4, 5, and 8 cardiac cycles. Three images were captured at each PI. All muscle and cardiac images were blindly analyzed using QLAB software (Philips Medical Systems) by W.C. and J.L., as described previously (5,7). MBF is derived from the product of MBV and MFV (i.e., MBF = MBV × MFV).
Biochemical analysis
Plasma cholesterol, HDL cholesterol, and triglycerides were assayed by the University of Virginia clinical chemistry laboratories. Plasma glucose was measured using a YSI glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was determined using the Immulite 2000 Automated Immunoassay Analyzer (Siemens Healthcare Diagnostics Inc., Deerfield, IL), and plasma FFAs were measured using an in vitro enzymatic colorimetric assay with a Wako HR Series NEFA-HR kit (Wako Diagnostics, Richmond, VA).
Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were performed using SigmaStat 3.1 software (Systat Software, Inc., Chicago, IL). Each subject served as his or her own control. Comparisons between measurements made before and after the insulin clamp within each study protocol and across all three studies were done using either paired Student t test, one-way ANOVA, or two-way repeated-measures ANOVA where appropriate. A P value < 0.05 was considered statistically significant.
RESULTS
All subjects were normotensive (systolic 128 ± 2 mmHg and diastolic 73 ± 2 mmHg) and had normal total cholesterol (172 ± 12 mg/dL), triglyceride (70 ± 9 mg/dL), LDL cholesterol (101 ± 8 mg/dL), and HDL cholesterol (59 ± 5 mg/dL) concentrations. Table 1 shows the plasma glucose, insulin, and FFA concentrations at baseline and after 2-h of insulin infusion. Insulin infusion during the control study raised plasma insulin concentrations to levels seen postprandially. This was associated with a significant decrease in plasma FFA concentrations. In the lipid protocol, lipid infusion raised basal plasma FFA concentrations by sixfold. Although it did not alter basal plasma glucose concentrations, it did increase basal plasma insulin levels by >60% (P < 0.001). Insulin infusion decreased plasma FFA concentrations by ∼1.3 mEq/L (31%, P < 0.001) during the lipid study. Salsalate pretreatment plus lipid infusion similarly did not alter plasma glucose concentrations. Plasma insulin concentrations trended up further (P = 0.08), but plasma FFA concentrations were significantly lower (P < 0.04) compared with the values obtained in the lipid alone study. Plasma FFA concentrations were similarly decreased after insulin infusion (24%, P = 0.001).
Plasma substrate concentrations during insulin clamp
| . | Control . | Lipid . | Salsalate + lipid . | |||
|---|---|---|---|---|---|---|
| Basal . | 120 min . | Basal . | 120 min . | Basal . | 120 min . | |
| Plasma glucose (mmol/L) | 5.1 ± 0.1 | 4.4 ± 0.1* | 5.1 ± 0.1 | 4.4 ± 0.1* | 5.0 ± 0.1 | 4.5 ± 0.1* |
| Insulin (pmol/L) | 29 ± 5 | 327 ± 22** | 48 ± 8# | 396 ± 77** | 74 ± 15## | 466 ± 43** |
| FFA (mEq/L) | 0.7 ± 0.1 | 0.2 ± 0.1** | 4.2 ± 0.4# | 2.9 ± 0.3** | 3.2 ± 0.3#& | 2.4 ± 0.3*** |
| . | Control . | Lipid . | Salsalate + lipid . | |||
|---|---|---|---|---|---|---|
| Basal . | 120 min . | Basal . | 120 min . | Basal . | 120 min . | |
| Plasma glucose (mmol/L) | 5.1 ± 0.1 | 4.4 ± 0.1* | 5.1 ± 0.1 | 4.4 ± 0.1* | 5.0 ± 0.1 | 4.5 ± 0.1* |
| Insulin (pmol/L) | 29 ± 5 | 327 ± 22** | 48 ± 8# | 396 ± 77** | 74 ± 15## | 466 ± 43** |
| FFA (mEq/L) | 0.7 ± 0.1 | 0.2 ± 0.1** | 4.2 ± 0.4# | 2.9 ± 0.3** | 3.2 ± 0.3#& | 2.4 ± 0.3*** |
*P < 0.0001, **P < 0.001, ***P = 0.001 vs. respective basal;
#P < 0.001, ##P < 0.005 vs. control basal;
&P < 0.04 vs. lipid control.
During the saline control study, the steady-state glucose infusion rates (GIRs) varied from 2.4 to 8.6 (average 5.6 ± 0.7) mg/kg/min across individuals. Figure 1B shows the GIRs during the entire 120 min of insulin infusion for all three protocols. Lipid infusion significantly decreased the GIR required to maintain euglycemia. The steady-state GIR decreased from 5.6 ± 0.7 to 4.5 ± 0.5 mg/kg/min (P < 0.001), and the overall GIR area under the curve declined by 20% (P < 0.007), indicating acute insulin resistance induced by lipid infusion. Salsalate treatment did not significantly alter the steady-state GIR (4.8 ± 0.6 mg/kg/min, P < 0.04 vs. control), but it did significantly improve the GIR area under the curve (back to the levels that were not statistically significantly different from the control study [P = 0.2]). This improvement in GIR was most evident during the first 60–90 min when the GIR initially tracked that of the control study. However, it then diverged and approached that observed in the lipid study by 120 min.
Figure 2A–C shows the changes of skeletal muscle microvascular parameters in all three studies. Similar to our previous reports (6,7), insulin potently increased muscle MBV and MBF without affecting MFV. Lipid infusion completely blocked these insulin-mediated increases in MBV and MBF. Salsalate pretreatment for 4 days prior to lipid infusion restored insulin-mediated increases near completely in MBV (P = 0.06) and completely in MBF.
Skeletal muscle MBV (A), MFV (B), and MBF (C) at baseline (open bar) and at end of 120-min insulin infusion (black bar). Compared with respective baseline, *P = 0.01, **P = 0.02.
Skeletal muscle MBV (A), MFV (B), and MBF (C) at baseline (open bar) and at end of 120-min insulin infusion (black bar). Compared with respective baseline, *P = 0.01, **P = 0.02.
Considering the cardiac microcirculation, Fig. 3A–C shows insulin infusion significantly increased myocardial MBV and MBF without changing MFV. As in skeletal muscle, these insulin effects were abolished by lipid infusion. With salsalate pretreatment, both MBV and MBF values post–insulin infusion were similar to those values seen in the control study. However, in this study there was an elevation of MBV (P = 0.1) and MBF (P < 0.03) seen prior to giving insulin, and there was no statistical significance between the pre- and postinsulin values.
Myocardial MBV (A), MFV (B), and MBF (C) at baseline (open bar) and at end of 120-min insulin infusion (black bar). Compared with baseline, *P = 0.01, **P = 0.001. Compared with control baseline, #P = 0.03.
Myocardial MBV (A), MFV (B), and MBF (C) at baseline (open bar) and at end of 120-min insulin infusion (black bar). Compared with baseline, *P = 0.01, **P = 0.001. Compared with control baseline, #P = 0.03.
CONCLUSIONS
The current study examined the effects of salsalate on FFA-induced microvascular and metabolic insulin resistance in humans, using a combination of contrast ultrasonography and hyperinsulinemic-euglycemic clamp methods. Our results indicate that high plasma concentrations of FFAs induce both microvascular and metabolic insulin resistance and that pretreatment with salsalate prevents FFA-induced muscle microvascular insulin resistance and attenuates whole body metabolic insulin resistance in a group of healthy young adults.
FFAs cause endothelial insulin resistance via activation of IKKβ (1). In cultured ECs, FFAs increase IKKβ activity and inhibit insulin-mediated eNOS phosphorylation and NO production (1). Salsalate is a potent anti-inflammatory drug (10), and our observation that salsalate prevents FFA-induced microvascular insulin resistance and attenuates whole body metabolic insulin resistance strongly suggests that in humans, FFAs cause microvascular insulin resistance via an inflammation-mediated pathway, which contributes to the metabolic insulin resistance. This is clinically significant because treatment with either aspirin or salsalate improves insulin resistance and glycemic control in animals (4,11,12), and clinical trials also have shown promising results. Indeed, studies in obese humans have shown that salsalate treatment significantly improves glycemia, decreases circulating C-reactive protein, and increases insulin-mediated glucose disposal (13,14). Salsalate treatment of patients with type 2 diabetes for 14 weeks significantly decreases glycated hemoglobin levels and improves circulating triglyceride and adiponectin concentrations (15).
In the current study, acute elevation of plasma FFA concentrations abolished insulin-mediated increases in skeletal and cardiac muscle microvascular recruitment and decreased insulin-stimulated whole body glucose disposal. This is in line with our previous reports in humans (6,7) and a previous report in rats (9). Strong evidence has confirmed that FFAs inhibit insulin signaling through the PI3-kinase pathway in both vasculature and muscle. In humans, high plasma concentrations of FFAs induce oxidative stress, impair endothelium-dependent vasodilation, blunt insulin-mediated vasodilation (16–18), and decrease muscle insulin receptor substrate-1-associated PI3-kinase activity and glucose transport (19). Increased FFA concentrations induce reactive oxygen species generation, which may reduce the bioavailability of NO and impair vascular reactivity. In addition, insulin has been shown to induce eNOS (20), and an insulin-resistant state may impair vasodilation by blocking insulin-mediated induction of eNOS. It is interesting that salsalate treatment prevented the lipid-induced decrease in whole body glucose disposal during the first 60–90 min only. Toward the last 30 min of clamp, insulin-stimulated glucose disposal rates were not different from the lipid group. That pretreatment with salsalate restored insulin response in muscle MBF suggests salsalate treatment completely prevented lipid-induced microvascular insulin resistance in muscle. Thus, the resolution of microvascular insulin resistance may have contributed to the early phase improvement of metabolic insulin sensitivity, whereas later muscle insulin resistance (without the vascular component) might have prevailed. Indeed, previous studies have demonstrated that insulin-mediated capillary recruitment contributes to 40% of insulin-mediated whole body glucose disposal and that vascular insulin resistance might be more important as a determinant of the rate of onset of insulin action (8,21).
Although salsalate treatment completely restored insulin responsiveness in skeletal muscle microcirculation, in the heart, there was no difference in either MBV or MBF before and after insulin infusion during the Intralipid plus salsalate admission. However, the absolute post–insulin infusion values were similar to those seen in the insulin control study. This was most likely secondary to the baseline increases in both MBV (P = 0.06) and MBF (P = 0.03) induced by lipid infusion in the setting of salsalate treatment. In the salsalate plus lipid protocol, basal MBV was 21 ± 9% higher than in the saline control subjects and only 4% less than the insulin-treated control subjects in the saline control protocol. Thus, the increase in MBV induced by lipid infusion after salsalate treatment may have preempted any insulin effect. The mechanisms underlying these increases in MBV and MBF in the heart are unknown. We and others have previously reported that lipid infusion increases basal cardiac (6) and total leg blood flow (18). It is interesting that the addition of salsalate to lipid infusion further enhanced basal cardiac MBF. The underlying mechanisms remain unclear. Given that plasma insulin concentrations increased significantly in the basal state, it is likely that both insulin and FFAs may have contributed to this increase in basal flow.
Inasmuch as salsalate is capable of increasing insulin secretion (14,22) and the plasma insulin trended higher during the salsalate plus lipid study than the lipid alone study, the improved microvascular and metabolic responses could be secondary to increased circulating insulin concentrations. Indeed, the significantly lower preclamp FFA concentrations are also suggestive of possible insulin effect. However, this probability is rather low because both the preclamp (P = 0.08) and postclamp (P = 0.51) insulin concentrations were not significantly higher during the salsalate plus lipid admission than the lipid alone admission. In addition, insulin increased muscle MBV only by ∼25% when its concentrations were raised by 10-fold in the current study.
The concentrations of FFAs used in the current study are higher than those observed in insulin-resistant states. We opted to use high concentrations of FFAs because in the past, we have demonstrated that FFAs at these concentrations blocked insulin- and mixed meal–induced muscle microvascular recruitment in healthy humans (6,7). Thus, it is very much likely that salsalate treatment could be more effective in the setting of low plasma concentrations of FFAs.
In conclusion, high plasma concentrations of FFAs cause muscle microvascular and whole body metabolic insulin resistance in healthy human adults. Salsalate treatment blocked FFA-induced microvascular insulin resistance, which was associated with a significant improvement in whole body insulin sensitivity. Thus, our data suggest that FFAs cause microvascular insulin resistance and dysfunction likely via an inflammation-mediated mechanism and that this contributes to metabolic insulin resistance in humans. Treatments aimed at inflammation inhibition may afford an opportunity to improve diabetes control and prevent cardiovascular complications in patients with type 2 diabetes.
Acknowledgments
E.J.B. has received National Institutes of Health (NIH) grants R01-DK-057878 and R01-DK-073759. Z.L. has received American Diabetes Association grants 1-11-CR-30 and 9-09-NOVO-11 and NIH Grant R01-HL-094722. The University of Virginia General Clinical Research Center has received a grant from the NIH (RR-00847).
No potential conflicts of interest relevant to this article were reported.
W.C., J.L., L.A.J., and D.E.F. researched data. E.J.B. contributed to discussion. Z.L. researched data and wrote the manuscript.
A part of this study was presented in the form of an oral presentation at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.