OBJECTIVE—This study, one of the first to address issues of pulmonary insulin delivery in smokers, compared pharmacokinetics of inhaled insulin delivered via the AERx insulin Diabetes Management System (iDMS) in nondiabetic cigarette smokers and nonsmokers.
RESEARCH DESIGN AND METHODS—In this randomized two-period crossover efficacy and safety trial in 27 nondiabetic smokers and 16 nonsmokers (18 men/25 women, mean age 28 years, mean BMI 23.0 kg/m2), subjects received single doses of inhaled insulin (33.8 IU) following overnight fasting on consecutive dosing days. On one dosing day, smokers smoked three cigarettes immediately before insulin administration (“acute smoking”); on the other dosing day, smokers had not smoked since midnight (“nonacute smoking”). After inhalation, 6-h serum insulin and serum glucose profiles were determined.
RESULTS—Pharmacokinetic results for evaluable subjects were derived from serum insulin profiles. The amount of insulin absorbed during the first 6 h after dosing (area under the exogenous serum insulin curve from 0 to 6 h [AUC(0–6 h)]) was significantly greater in smokers (63.2 vs. 40.0 mU · l−1 · h−1, P = 0.0017); peak concentration was both higher and earlier in the smokers (maximal serum concentration of insulin [Cmax] 42.0 vs. 13.9 mU/l, P < 0.0001; time to maximal serum concentration of insulin [tmax] 31.5 vs. 53.9 min, P = 0.0003). The estimated intrasubject variability of AUC(0–6 h) was 13.7 and 16.5% for nonsmokers and smokers, respectively. No safety issues arose.
CONCLUSIONS—Absorption of inhaled insulin via the AERx iDMS was significantly greater in smokers, with a higher AUC(0–6 h) and Cmax and a shorter tmax. Intrasubject variability of AUC(0–6 h) was low and similar in nonsmokers and smokers. These data prompt more extensive investigation of inhaled insulin in diabetic smokers.
Intensive insulin therapy is the cornerstone of glycemic control in diabetic patients, reducing complications in type 1 and type 2 diabetic subjects (1,2). Despite improvements in regimens, inconvenient multiple insulin injections can pose a barrier to good glycemic control. Alternative routes for insulin administration have been determined, with inhaled insulin emerging as a viable alternative (3,4). Advances in aerosol technology have resulted in the efficient delivery of smaller-sized particles (for example, AERx insulin Diabetes Management System [iDMS], Exubera, Aerodose, and Technospheres). The clinical performance, reproducibility, and patient satisfaction with these emerging systems are currently being investigated (3,5–7) based on the insulin profiles seen using this route of administration, which resemble profiles of rapid-acting analogs (4,8).
Patients with diabetes have similar smoking habits to smokers without diabetes, with as many as 17–26% of diabetic patients smoking cigarettes (9). The long-term adverse effects of cigarette smoking are well established (10), and smoking has additional acute effects on the lungs, increasing the permeability of the alveolar-capillary barrier in animal models (11) and humans (12). The effects of these smoking-related pulmonary changes on the delivery and absorption of inhaled drugs are not yet fully understood. Smoking dramatically increases the rate of absorption of the β agonist terbutaline (13) and decreases subcutaneous absorption of insulin, increasing dosage requirements (14). Smoking also acutely impairs insulin action leading to insulin resistance (15).
Because insulin has a narrow therapeutic index, the AERx iDMS, an inhalation-activated system with insulin strips, has been developed to enable liquid aerosols of bolus insulin to be delivered into the deep lung. It emits a fine particle aerosol (mass median aerodynamic diameter of 2–3 μm) from single-use insulin strips by extruding the prepacked solution through hundreds of precisely laser-evaporated holes in a single-use nozzle. The dose will be selected in AERx units in the final version of the AERx iDMS (1 AERx unit = ∼1 IU given subcutaneously) and 1-unit increments will be possible, with a clear dose response as shown in previous trials (8,16). Trials have demonstrated the efficacy and reproducibility of insulin administered from AERx iDMS compared with subcutaneous delivery, with no safety concerns or serious adverse events (8,16).
The present study was performed with a prototype of AERx iDMS to evaluate the pharmacokinetics, safety, and tolerability of inhaled insulin delivered to a smoking population, to assess the acute effects of smoking, and to compare these findings with those from healthy nonsmoking volunteers. This is one of the first studies to address these issues of pulmonary insulin delivery in smokers.
RESEARCH DESIGN AND METHODS
The trial was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee, and all subjects gave their written informed consent before entering the trial.
Volunteers
Healthy volunteers were current smokers or nonsmokers of either sex, with a mean age of 27.7 ± 5.4 years and a mean BMI of 23.4 ± 2.2 kg/m2 for smokers and 23.0 ± 1.7 kg/m2 for nonsmokers (Table 1). A total of 43 eligible volunteers had inhaled insulin administered, and these individuals comprised 27 smokers and 16 nonsmokers. Smokers were defined as smoking, on average, ≥10 cigarettes a day for at least 5 years. Nonsmokers were defined as not having smoked tobacco for at least the previous 3 years and as having an average total smoking history of ≤7 pack-years (e.g., one pack of 20 cigarettes a day for 7 years). Nonsmoker and smoker status were also confirmed by the absence or presence, respectively, of urine cotinine.
All volunteers included in the trial had normal pulmonary function, as determined by pulmonary function tests at screening. Subjects with active, chronic, or a history of pulmonary disease were excluded.
Protocol and methods
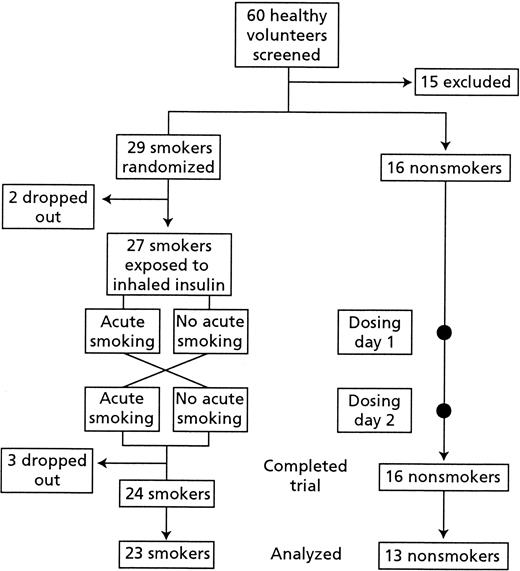
In this single-center open-label trial (Fig. 1), nonsmokers and smokers received a single dose of inhaled insulin on each of two consecutive dosing days. Within the nonsmoking group, subjects experienced identical conditions on both dosing days.
A two-period crossover design was applied within the smoker group. This allowed smokers to act as their own control and facilitated assessment of the effects of acute smoking before insulin inhalation. Smokers were randomized to either not smoke from midnight until the end of dosing day 1 (“nonacute smoking”), and then on dosing day 2 to smoke three cigarettes during the 30 min before insulin dosing (“acute smoking,” to simulate the extreme situation), or vice versa. Subjects were hospitalized and test conditions were identical on both dosing days. Smoke inhalation did not differ between smoking groups (acute and nonacute smoking), and subjects used their own cigarettes and inhalation habits.
In the morning of each dosing day and after an overnight fast, a single dose of inhaled human soluble insulin in disposable insulin strips (67.5 IU/strip; Novo Nordisk A/S; Aradigm Corporation) was administered to each volunteer via a representative prototype AERx iDMS (Novo Nordisk A/S; Aradigm Corporation). The insulin was administered at a revised dose of 33.8 IU (half a strip; previously calculated to have an effect corresponding approximately to a single subcutaneous dose of 4.4 IU with this prototype) (8).
Because of pronounced hypoglycemia in one of the first two subjects tested, four subjects initially received a lower insulin dose of 22.5 IU. The protocol was then amended when results from another study became available demonstrating a lower relative bioavailability of inhaled insulin than perceived on original dose selection. As a result of this new data, all subsequent subjects finally received a revised dose of 33.8 IU. To ensure safety at this higher dose, all subjects in whom intolerable signs and symptoms of hypoglycemia (and/or duplicate blood glucose readings on a HemoCue Glucometer of ≤2.0 mmol/l) were observed were treated with intravenous glucose infusion to raise their blood glucose level to 2.5 mmol/l or a level necessary to relieve symptoms, whereas the blood sampling continued as planned. This glucose infusion stymied interpretation of pharmacodynamic parameters.
After inhalation of the trial drug, subjects held their breath for 10 s and then exhaled slowly. No reference therapy was used, but strips containing sterile water were used for inhalation training purposes.
Blood sampling for serum insulin and serum glucose was carried out at preplanned intervals before the dose and for 6 h after insulin administration. Smoking was not permitted during the sampling period. Serum glucose was determined using a glucose oxidase method. Serum insulin and C-peptide were both measured using commercially available ELISA methods (Dako, Cambridgeshire, U.K.). Exogenous insulin concentration was derived as
where the initial insulin and C-peptide concentrations were computed as the average of measurements up to the time of administration (17).
All laboratory analyses were performed in a central laboratory (Medi-Lab A/S, Copenhagen, Denmark). In the case of hypoglycemic symptoms, blood glucose for the safety analysis was measured in duplicate at the clinic using a HemoCue B-Glucose Analyser (HemoCue AB, Angelholm, Sweden).
In addition, pulmonary function tests were performed at baseline, on the predosing day, and before and 6 h after the insulin administration on both dosing days. These included forced expiratory volume during the first second (FEV1), forced expiratory volume percent (calculated as FEV1/forced vital capacity [FVC] and expressed as a percentage), and FVC (Table 1). Lung function variables were expressed as a percentage of predicted normal values.
Safety assessments included frequency of adverse events, pulmonary function tests, physical examination, electrocardiogram, vital signs, and standard laboratory safety analyses.
Statistical analysis
The pharmacokinetic analyses were based on 23 smokers and 13 nonsmokers with evaluable insulin data.
The primary pharmacokinetic end point, area under the exogenous serum insulin curve from 0 to 6 h [AUC(0–6 h)], was log-transformed and analyzed in an ANOVA model with smoker/nonsmoker groups and acute smoking/no smoking as fixed effects and subject as random effect. The areas under the curve were calculated using the trapezoidal method. The contrasts within this model (smoking vs. no smoking; acute vs. nonacute smoking) were tested for significance, and the difference with 95% confidence limits was calculated. The intra- and intersubject coefficients of variation (CVs) of AUC(0–6 h) were estimated separately for smokers and nonsmokers, and a 95% CI for the intrasubject CV was constructed. Nonsmokers experienced two dosing days under the same conditions. For the smoking group, data from the nonacute and acute dosing days were used. Assuming a common effect, the estimation of intrasubject CV in smokers included adjustment for the systematic effect of acute smoking versus nonacute smoking. The final CV estimate therefore represents the intrasubject variability that can be attributed to chronic or long-term smoking.
The secondary pharmacokinetic end points—maximal serum concentration of insulin (Cmax) and time to maximal serum concentration of insulin (tmax), initial rate of increase of serum insulin concentration, mean residence time (MRT), and terminal elimination rate constant—were analyzed in a similar way to the AUC(0–6 h). Inter- and intrasubject CVs of Cmax and tmax were estimated for smokers and nonsmokers. The safety evaluation was based on the safety analysis group (all subjects exposed to trial product [n = 43]).
RESULTS
Demographic characteristics
The two groups were well matched for age, sex, and BMI at baseline (Table 1). Of the 43 subjects exposed to the trial drug, 18 were men and 25 were women, with a mean age of 28 years and a mean BMI of 23 kg/m2. Within the smoking group, the mean duration of smoking was 13 years. Three smokers were withdrawn from the trial after dosing (Fig. 1). Of these, two were withdrawn because of hypoglycemia (before the protocol was amended), and one was withdrawn because of problems with insertion of the cannula. All withdrawers were excluded from the concentration analyses. Additionally, four other subjects were excluded from the serum insulin analyses. Of these, one smoker and two nonsmokers had received the lower dose and thus were excluded, and one nonsmoker was excluded because of analytical problems with the samples.
Pharmacokinetic results
Pharmacokinetic data were derived from exogenous serum insulin profiles obtained on each dosing day by correcting total insulin based on C-peptide measurements (Fig. 2), comparing area under the insulin-concentration time curve and other pharmacokinetic parameters in the different study groups.
Smoking versus nonsmoking.
More insulin was absorbed in the smoking group than in the nonsmoking group, as demonstrated by the mean AUC(0–6 h) for serum insulin, which was ∼60% greater for smokers than nonsmokers (63.2 vs. 40.0 mU · l−1 · h−1, P = 0.0017) (Table 2). The mean exogenous insulin profiles (Fig. 2) demonstrated clear differences between smokers and nonsmokers during the first 100 min after the dose. After this, the differences were minimal.
Cmax was approximately three times higher among smokers than nonsmokers (P < 0.0001) (Table 2). Absorption of insulin appeared to be significantly faster in smokers than in nonsmokers and tmax was 22 min shorter (P = 0.0003) (Table 2). The initial rate of increase of serum insulin concentration (the estimated slope of the serum insulin profile during the period from 0 to 15 min after dosing) was significantly higher (∼3.7 times) in smokers than in nonsmokers (P < 0.0001).
The more rapid absorption of insulin in smokers than in nonsmokers was also evident when comparing values of MRTs and terminal elimination rate constants. The MRT in the smoker group was less than half of that in the nonsmoking group (P < 0.0001), and the apparent elimination rate constant of exogenous serum insulin was almost twice as high in smokers as in nonsmokers (P = 0.0019). Because absorption is the rate-limiting factor for disappearance of insulin from serum, the faster apparent elimination in smokers is more a reflection of the increased absorption rate than an indication of a difference in the elimination process between smokers and nonsmokers.
Acute versus nonacute smoking.
Within the smoking group, mean AUC(0–6 h) was significantly reduced (by 22%) after acute smoking compared with nonacute smoking (P < 0.0001). The mean exogenous insulin profiles (Fig. 2) showed differences between acute and nonacute smoking, but again only during the first 100 min after the dose.
Cmax was 23% lower after acute smoking compared with nonacute smoking (P = 0.0013). No effect of acute smoking was seen for tmax (Table 2).
The initial rate of increase of serum insulin concentration was significantly (33%) lower after acute smoking compared with nonacute smoking (P = 0.0008). The terminal elimination rate constant of exogenous serum insulin appeared to be similar on both occasions.
Intrasubject variability.
The intrasubject variability, as measured by CV (95% CI) of AUC(0–6 h) was similar for smokers and nonsmokers, being 16.5% (12.7–23.6) and 13.7% (9.8–22.6), respectively.
Intrasubject variability of Cmax (95% CI) was similar for both smokers and nonsmokers, being 23.8% (18.3–34.1) and 26% (18.6–42.9), respectively, whereas tmax values were 59 and 43%, respectively.
Pharmacodynamic results
This was primarily a pharmacokinetic study. However, serum glucose was measured in the trial (Fig. 2), but because of intervention with glucose infusion in 13 subjects (12 smokers, 1 nonsmoker), as per protocol, no formal analyses could be made and no conclusions were drawn.
Safety
A total of 13 subjects (12 smokers, 1 nonsmoker) underwent glucose infusion because of minor hypoglycemia, as per protocol. There were 16 smokers who experienced hypoglycemia (11 infused) on the nonacute dosing day and 13 smokers on the acute dosing day (again, 11 were infused). A total of 12 smokers received glucose infusion on one or both of the days; one nonsmoker received glucose infusion on both days. Note that Cmax was reached before glucose infusion and was unaffected by subsequent insulin secretion.
None of the subjects withdrew because of study-related adverse events. A total of 17 (40%) subjects experienced adverse events; all were mild or moderate and evenly distributed between groups. Headache was the most frequently reported adverse event (n = 9) but was likely to be due to fasting or, in the smokers, abstinence from smoking. Headache and hypoglycemia coincided on one occasion in only one subject. There were no clinically relevant changes in mean or individual pulmonary function test results in the two groups from screening to the end of the trial. No clinically relevant changes in other safety parameters were reported.
CONCLUSIONS
This study, to investigate pulmonary insulin absorption in nondiabetic smoking volunteers, highlights the issue in smokers of an apparent increased absorption compared with nonsmokers when dosed with pulmonary insulin via the AERx iDMS, an effect that is somewhat blunted by acute smoking.
Smokers comprise a substantial proportion of the diabetic population; therefore, it is essential to obtain an insight into pharmacokinetic patterns for inhaled insulin in these individuals. The present study showed a near physiological insulin profile gained with the AERx iDMS in cigarette smokers.
Interestingly, although administration of inhaled insulin is highly reproducible with the AERx iDMS system in smokers in this trial, and there is no reason to suggest that this would not also be the case in patients with diabetes, the major challenge would be selection of an appropriate dose depending on smoking pattern.
Smoking versus nonsmoking
The overall amount of insulin absorbed and peak insulin concentration was significantly greater and insulin absorption was significantly faster in the smoking population than in the nonsmoking population. A significantly higher elimination rate constant and shorter MRT for insulin was observed in smokers.
It is well established in both animal models (11) and humans (12) that cigarette smoke increases the permeability of the alveolar-capillary barrier. Postulated mechanisms for these physiological changes include immunological modifications (18), increased blood perfusion (19), surfactant antioxidant depletion (20), or disruption in surfactant function (21). These mechanisms may also be occurring in smokers inhaling insulin and would explain the insulin profiles seen.
Increased metabolism of drugs in smokers, possibly due to the induction of drug-metabolizing enzymes, has also been demonstrated (14). Any augmented metabolism of insulin would reduce its action and may necessitate a dosage adjustment.
It is important to note that, despite an apparent increase in absorption, there may not necessarily be a concomitant increase in insulin action. A study using the Spiros system (Eli Lilly) has shown that relative insulin resistance in smokers may lessen the response to a greater insulin peak (15). Cigarette smoke has been found to acutely impair insulin action and to lead to insulin resistance (22,23) and glucose intolerance (24) in healthy smokers and exacerbate insulin resistance in patients with type 2 diabetes (25). Because pharmacodynamic parameters were not specifically addressed in this trial, we are unable to comment at this time on the likely glucose-lowering effect produced by inhaled insulin in smokers.
Several issues must be addressed if smokers are to be prescribed inhaled insulin via the AERx iDMS. The increased absorption of inhaled insulin in smokers may have a considerable influence on dose and timing of delivery in relation to meals and cigarette smoking. The rapidly emerging high peaks in insulin concentration observed among smokers would need to be considered when formulating dosing guidelines in the smoking population. There is evidence to suggest that there is improvement in alveolar-capillary barrier function within a week after stopping smoking (26), which could help to normalize inhaled insulin absorption after smoking cessation. Although the chosen dose could possibly be reduced to accommodate the effect of smoking, the unique 1 AERx unit increment would no longer be useful, because adjusting the dose by 1 AERx unit would result in a serum insulin increment equivalent to between 2 and 3 IU.
Acute versus nonacute smoking
Within smoking subjects, acute smoking just before insulin inhalation blunted the enhanced absorption of insulin significantly compared with nonacute smoking in the same group. However, no difference in time to peak concentration was observed.
The reasons why acute smoking should limit physiological increases in inhaled insulin absorption compared with nonacute smoking are unclear. Smoking is known to reversibly constrict the smooth muscle of the airways, which may influence the distribution, and absorption, of inhaled insulin on acute smoking. Nicotine may also cause cutaneous vasoconstriction, and this has been shown to delay the absorption of subcutaneously injected insulin (27). As intense exposure to tobacco smoke gives rise to the activation of leukocytes (18), it is also possible that this could cause insulin to be degraded in the alveoli. Even if this blunting effect turns out to be constant for any given patient, the dose of the AERx iDMS given would have to be adjusted for this smoking pattern.
Subjects adopted the same inhalation habits for both acute and nonacute smoking situations and were not instructed to do otherwise; therefore, it is unlikely that variable inhaling habits explain the difference in insulin absorption.
Intrasubject variability
Intrasubject variability of extent of insulin absorption was low and similar in smokers and nonsmokers (16.5 and 13.7%, respectively), which is encouraging and implicates an inherent reliability within the AERx iDMS system. This variability compares favorably with that seen after subcutaneous injection of insulin in a recent trial with the AERx iDMS showing similar low variability of insulin and glucose after inhaled and subcutaneous insulin administration to type 1 diabetic patients (7). This might be further improved by patient education.
The introduction of acute smoking did not appear to alter the reliability of insulin dosing in subjects.
Clinical implications
The data presented here support the consistency of AERx iDMS, with its unique “breath control” activation mechanism (extrusion of insulin only when inspiratory flow rate is optimal for deep lung delivery, to minimize any influence of patient breathing technique). The data also confirm that the system remains reproducible in a smoking population.
The study highlighted that different smoking patterns (e.g., nonacute vs. acute smoking), between and within individuals, could theoretically influence dose requirements of inhaled insulin. Because inhaled insulin is likely to be popular among smokers and nonsmokers alike, it is clear that greater attention needs to be paid to this subsection of the population, especially if the pulmonary route of drug delivery is to be exploited to its full potential. We await further investigation of inhaled insulin absorption in smokers and how this compares to subcutaneous insulin absorption in the same patients, to clarify the efficacy and safety of the AERx iDMS in the daily life of a diabetic patient who smokes. Furthermore, the issue of potential long-term effects of inhaled insulin in smokers and nonsmokers alike warrants further attention.
In summary, the present study has shown that dosing with AERx iDMS is highly reproducible in both the smoking and nonsmoking population. It must be emphasized, however, that this pharmacokinetic study was performed in nondiabetic subjects using relatively low doses of insulin. The use of inhaled insulin in a more appropriate clinical setting must of course be further investigated before clear guidelines can be given for this patient group.
Mean exogenous serum insulin curves and glucose profiles for smokers and nonsmokers. NB: intravenous glucose infusion was initiated if a subject’s blood glucose reached 2.0 mmol/l. The vertical line at 28 min marks the time of the first glucose infusion.
Mean exogenous serum insulin curves and glucose profiles for smokers and nonsmokers. NB: intravenous glucose infusion was initiated if a subject’s blood glucose reached 2.0 mmol/l. The vertical line at 28 min marks the time of the first glucose infusion.
Baseline characteristics
| . | Smokers . | Nonsmokers . |
|---|---|---|
| Sex (M/F) | 11/16 | 7/9 |
| Age (years) | 27.7 ± 5.7 | 27.7 ± 5.2 |
| Weight (kg) | 71.7 ± 10.0 | 71.3 ± 7.4 |
| BMI (kg/m2) | 23.4 ± 2.2 | 23.0 ± 1.7 |
| FVC (% predicted) | 103 ± 10.2 | 103 ± 10.0 |
| FEV1 (% predicted) | 97.9 ± 9.2 | 96.0 ± 8.3 |
| FEV% (% predicted) | 94.6 ± 7.6 | 93.0 ± 6.6 |
| . | Smokers . | Nonsmokers . |
|---|---|---|
| Sex (M/F) | 11/16 | 7/9 |
| Age (years) | 27.7 ± 5.7 | 27.7 ± 5.2 |
| Weight (kg) | 71.7 ± 10.0 | 71.3 ± 7.4 |
| BMI (kg/m2) | 23.4 ± 2.2 | 23.0 ± 1.7 |
| FVC (% predicted) | 103 ± 10.2 | 103 ± 10.0 |
| FEV1 (% predicted) | 97.9 ± 9.2 | 96.0 ± 8.3 |
| FEV% (% predicted) | 94.6 ± 7.6 | 93.0 ± 6.6 |
Data are means ± SD. FEV1, forced expiratory volume during the first second; FEV%, forced expiratory volume %; FVC, forced vital capacity.
Pharmacokinetic parameters in nonsmokers and smokers and in acute and nonacute smoking
| . | n . | AUC(0–6 h) (mU · l−1 · h−1) . | Cmax (mU/l) . | tmax (min) . |
|---|---|---|---|---|
| Nonsmoking mean | 13 | 40.0 | 13.9 | 53.9 |
| Smoking mean | 23 | 63.2 | 42.0 | 31.5 |
| Mean ratio | — | 1.58 | 3.02 | −22.4* |
| 95% CI | — | 1.20 to 2.08 | 1.94 to 4.70 | −34.2 to −10.6 |
| P | — | 0.0017 | <0.0001 | −0.0003 |
| Acute smoking mean | 23 | 55.8 | 36.8 | 30.0 |
| Nonacute smoking mean | 23 | 71.5 | 47.8 | 33.3 |
| Mean ratio | — | 0.78 | 0.77 | −3† |
| 95% CI | — | 0.70 to 0.86 | 0.67 to 0.89 | −14 to 7.16 |
| P | — | <0.0001 | 0.0013 | 0.5340 |
| . | n . | AUC(0–6 h) (mU · l−1 · h−1) . | Cmax (mU/l) . | tmax (min) . |
|---|---|---|---|---|
| Nonsmoking mean | 13 | 40.0 | 13.9 | 53.9 |
| Smoking mean | 23 | 63.2 | 42.0 | 31.5 |
| Mean ratio | — | 1.58 | 3.02 | −22.4* |
| 95% CI | — | 1.20 to 2.08 | 1.94 to 4.70 | −34.2 to −10.6 |
| P | — | 0.0017 | <0.0001 | −0.0003 |
| Acute smoking mean | 23 | 55.8 | 36.8 | 30.0 |
| Nonacute smoking mean | 23 | 71.5 | 47.8 | 33.3 |
| Mean ratio | — | 0.78 | 0.77 | −3† |
| 95% CI | — | 0.70 to 0.86 | 0.67 to 0.89 | −14 to 7.16 |
| P | — | <0.0001 | 0.0013 | 0.5340 |
Shown are estimated means, ratios, CIs, and P values based on ANOVA with log-transformed response, adjusted for sex, period, and acute smoking.
For tmax based on mean difference (smokers minus nonsmokers);
tmax based on mean difference.
Article Information
The study was performed with financial support from Novo Nordisk A/S.
References
Address correspondence and reprint requests to Associate Professor Anders Himmelmann, MD, Department of Clinical Pharmacology, Sahlgrenska University Hospital, SE-413 45 Gothenburg, Sweden. E-mail: anders.himmelmann@pharm.gu.se.
Received for publication 14 August 2002 and accepted in revised form 11 November 2002.
A.H.P. and U.L.D. hold stock in Novo Nordisk A/S. P.W. has received honoraria for speaking engagements from and serves on an advisory panel for Novo Nordisk A/S.
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.