To evaluate two widely used control algorithms for an artificial pancreas (AP) under nonideal but comparable clinical conditions.
After a pilot safety and feasibility study (n = 10), closed-loop control (CLC) was evaluated in a randomized, crossover trial of 20 additional adults with type 1 diabetes. Personalized model predictive control (MPC) and proportional integral derivative (PID) algorithms were compared in supervised 27.5-h CLC sessions. Challenges included overnight control after a 65-g dinner, response to a 50-g breakfast, and response to an unannounced 65-g lunch. Boluses of announced dinner and breakfast meals were given at mealtime. The primary outcome was time in glucose range 70–180 mg/dL.
Mean time in range 70–180 mg/dL was greater for MPC than for PID (74.4 vs. 63.7%, P = 0.020). Mean glucose was also lower for MPC than PID during the entire trial duration (138 vs. 160 mg/dL, P = 0.012) and 5 h after the unannounced 65-g meal (181 vs. 220 mg/dL, P = 0.019). There was no significant difference in time with glucose <70 mg/dL throughout the trial period.
This first comprehensive study to compare MPC and PID control for the AP indicates that MPC performed particularly well, achieving nearly 75% time in the target range, including the unannounced meal. Although both forms of CLC provided safe and effective glucose management, MPC performed as well or better than PID in all metrics.
Introduction
Numerous studies have evaluated the efficacy of controlled artificial pancreas (AP) devices for persons with type 1 diabetes (T1DM). These studies were performed in camps, in a clinic setting, in supervised outpatient settings, and at subjects’ homes (1–9). Comparing results across these studies to determine which AP control algorithm works best has been difficult due to the many variables inherent in T1DM management. For example, clinical trials can be held in different locations; involve subjects of different ages and lifestyles; use different devices, algorithms, and settings; and use single or multihormonal therapy. Other factors include different (or no) exercise periods, announced (bolused) versus unannounced meals, overnight-only or 24-h trials, and prebolus for meals versus bolus at mealtime.
To date, no clinical trial has directly compared the performance of model predictive control (MPC) and proportional integral derivative (PID) control algorithms for AP under identical conditions in a randomized crossover design. Similarly, most studies have not intentionally stressed the limits of AP systems to see how well they perform under nonideal conditions. For instance, some protocols have subjects prebolus as much as 30 min prior to meals (10). While this strategy improves results (11), it does not test operation of the system under realistic conditions, such as when patients bolus at mealtime, or forget to bolus, as can happen in unsupervised home settings, where up to half of adults report omission of boluses (12).
Anticipating that challenges may occur with practical use of AP technologies outside of clinical research protocols, we performed the first randomized, crossover study to expressly compare MPC and PID for AP under identical, nonideal conditions in 27.5-h sessions. Challenges in the study were designed to model the realistic use of a future AP, including no prior optimization of subjects’ insulin pump settings, overnight control (midnight to 7:00 a.m.) after a 65-g dinner, response to a 50-g breakfast (both bolused at mealtime), and an unannounced 65-g lunch to evaluate the response to a missed meal bolus scenario.
Research Design and Methods
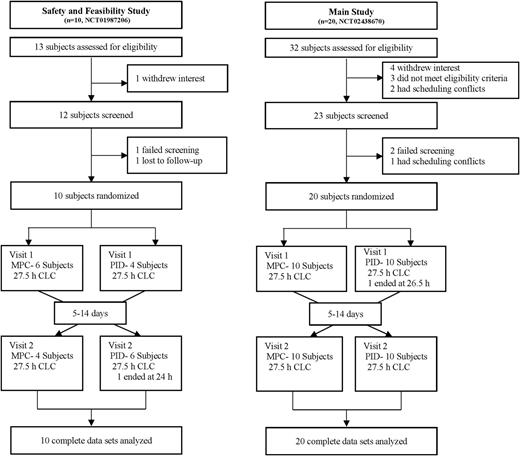
The study was performed at the William Sansum Diabetes Center. Design of the control algorithms and engineering of the AP device were done at the Department of Chemical Engineering at the University of California, Santa Barbara. Due to the numerous challenges in the study and the potential for hyper- and hypoglycemia, an initial safety and feasibility study was performed (n = 10) (NCT01987206, clinicaltrials.gov) with hourly YSI 2300 Stat (Yellow Springs Instruments, Yellow Springs, OH) glucose monitoring and close clinical supervision for the 27.5 h of closed-loop control (CLC). Based on the results of the pilot study (13), we improved safety of the control algorithms and subsequently performed this study (n = 20) (NCT02438670, clinicaltrials.gov) with less stringent clinical monitoring (described below). The primary end point was percent time in glucose range 70–180 mg/dL (safe glucose range) by continuous glucose monitoring. Secondary end points included time spent in the hypoglycemic range (<70 mg/dL), time spent in the hyperglycemic range (>180 mg/dL), and the need for outside intervention to prevent hypo- or hyperglycemia. The study compared results from both CLC strategies (MPC and PID). These end points were determined for the entire study: the 5-h postprandial meal responses for both announced meals and the unannounced meal and for the nocturnal period (12:00 a.m.–7:00 a.m.). The trial was approved by both the U.S. Food and Drug Administration and the Cottage Health System Institutional Review Board.
Subjects and Closed-Loop System
A total of 30 subjects completed the studies, as summarized in Fig. 1. Ten subjects completed the pilot safety and feasibility trial. An additional 20 subjects completed the current study. Inclusion criteria were age 21–65 years with diagnosed T1DM for more than 1> year, use of continuous subcutaneous insulin infusion pump therapy for at least 6 months, and an HbA1c between 5.0% (31 mmol/mol) and 10.0% (86 mmol/mol). Exclusion criteria were diabetic ketoacidosis or severe hypoglycemia within the past year, abnormal lab findings, pregnancy, current participation in another study, and comorbidities that would affect the safety of the subject or prevent completion of the study. Informed consent was obtained prior to all study procedures. Recruitment and enrollment of subjects were in compliance with ethics standards set by the Santa Barbara Cottage Health System Institutional Review Board.
The subjects wore two Dexcom G4 Platinum continuous glucose monitors (CGM) (Dexcom, Inc., San Diego, CA), placed at least 48 h prior to study visits. Subjects calibrated both CGMs 30 min before all meals and at bedtime throughout the study session. During CLC, subjects wore the Animas OneTouch Ping Glucose Management System (Animas Corporation, Westchester, PA) continuous subcutaneous insulin infusion pump. Wireless communication among the system components occurred with our portable artificial pancreas system (pAPS), version 1.9.8.1, running on a Windows tablet computer (14). The pAPS used the subjects’ open-loop basal rates, insulin-to-carbohydrate ratios (CR), and insulin sensitivity factors to initialize both CLC sessions.
Study Design
Each subject was randomly assigned to the MPC or the PID arm of the study for 27.5 h in a supervised outpatient suite. Each subject returned 5–14 days after the first session to cross over to the second arm of the study (Fig. 1). Prior to the start of each session, subjects were given a supervised lunch while still on sensor-augmented pump (SAP) therapy and observed to ensure equitable starting conditions for each CLC session. One primary CGM was selected for use with the pAPS at the start of each CLC session, and the secondary CGM was kept as a backup in case of sensor failure (occurred for two subjects). Two announced meals (dinner, 65 g, at ∼7:00 p.m. and breakfast, 50 g, at ∼7:00 a.m.) and an unannounced meal (lunch, 65 g, at ∼1:00 p.m.) were provided. The contents of the dinner and next day’s lunch were identical for each subject in both arms of the study, although the contents of each meal varied across subjects. All meals were separated by at least 5 h to ensure that the effects of the previous meal bolus were no longer present by the next meal. Boluses for announced meals under CLC were given at mealtime based on the subject’s CR. Similar to our previous study designs (1), the CLC system modified the mealtime bolus based on CGM value at the time of the meal as follows: for CGM <140 mg/dL, 80% of the bolus calculated using the subject’s own CR was delivered; for CGM ≥140 mg/dL, the full bolus was given with an additional correction based on the subject’s own correction factor to 140 mg/dL. No interventions were made to optimize glucose levels prior to bedtime. Capillary fingerstick glucose measurements were performed 30 min prior to meals, 2 h after meals, at bedtime, and when prompted by the University of California, Santa Barbara health monitoring system (HMS) (6). Subjects were discharged at ∼7:00 p.m. on the second day of the study.
Safety
The AP incorporated the HMS, an algorithm that added an independent safety layer (15). The HMS was independent from the AP control algorithms and advised subjects to ingest 16 g carbohydrate after confirmation of blood glucose (BG) by fingerstick to prevent impending hypoglycemia that could not be prevented by controller action alone. Hypoglycemia treatments resulting from HMS alerts were counted as hypoglycemic events in our analysis, as previously recommended (16). The goal of the AP device was to operate without outside intervention, apart from meal boluses and HMS alerts. The pAPS system, with the HMS, was active for 99.6% of the entire trial duration.
Control Algorithm Design
In comparison of multiple control algorithms, it is imperative to reproduce the same test conditions. That is, the algorithms should be designed using the same available information and evaluated under identical conditions. It was reported by Percival et al. (17) that MPC with a specific choice of design parameters yields a control law that is functionally identical to a particular PID design; i.e., a PID controller response can be recreated by a suitable MPC design. MPC and PID (including internal model control) control algorithms have been designed for the AP and compared in silico in prior studies (18,19). However, in those studies, identical models were not used in both algorithms, and the algorithms were not balanced for a detailed comparison regarding things like insulin on board. Thus, we built upon the results of this prior literature and our own recent work to design balanced controllers for comparison in clinical trials (20).
Both the MPC and PID controllers were derived using model-based design methods and the same empirical model of BG-insulin dynamics (20), with a set point of 110 mg/dL. A novel model personalization scheme that takes into account each subject’s basal profile and responds to individual time-dependent variation in insulin sensitivities was used within this model (21). MPC is not a specific design but a general control paradigm. MPC comes in many flavors and is extremely flexible, with the capability to incorporate zone-based objectives and asymmetric costs (22,23). This flexibility has also allowed MPC to be used for dual hormone approaches to AP (2).
To facilitate an equitable comparison of MPC and PID, we opted to use a generic MPC strategy that does not incorporate these additional features. Each controller was modified to compensate for insulin stacking using methods that have been verified in clinical trials (insulin feedback method for PID control and insulin on board for MPC) (24,25). The controllers were tuned to have the same level of aggressiveness and were evaluated under identical simulation conditions using the U.S. Food and Drug Administration–accepted Universities of Virginia/Padova metabolic simulator with 100 in silico adult subjects (26).
Additional details about the derivation and development of these control algorithms have previously been published (21,27) and are also available in Supplementary Data.
Statistical Analysis
Power analysis was performed based on the results of the pilot safety and feasibility study for the proposed end points (% time in the safe glucose range). With the criterion for significance (α) set at 0.05, analysis indicated a minimum sample size of 18 subjects to achieve a power of 80%. We chose to enroll 20 subjects in the current study. All glucose data are reported as mean (SD) unless otherwise specified. Missing CGM data were linearly interpolated, and missing CGM data at the end of the study were imputed to be unchanged from the final time point until the expected end of the study (28). This imputation was conducted primarily to account for subjects who ended the study early for persistent hyperglycemia after the unannounced meal. The imputation did not alter the results, which is similar to other studies where missing CGM data were imputed (29).
The differences between each controller group for each metric were compared using paired two-sample t tests. MATLAB 2015B software (Mathworks, Inc., Natick, MA) was used for all analyses.
Results
Subject Characteristics
Ten subjects completed the pilot study (6 female and 4 male). Twenty additional subjects (13 and 7 male) completed the current study. Subject demographics are summarized in Supplementary Table 1.
Glycemic Control
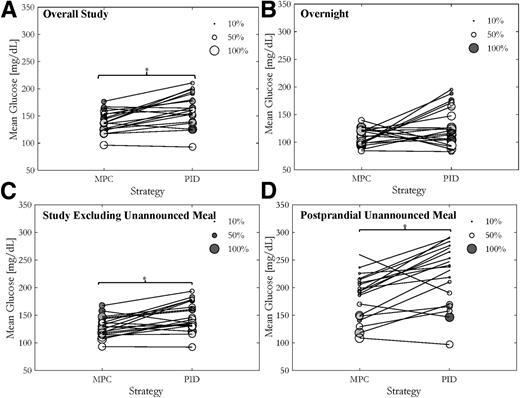
Results are reported for all 20 subjects who completed the study. Cumulative distributions of glucose levels comparing MPC and PID are shown in Fig. 2A. For the overall study, MPC showed a statistically significant greater percent time in the safe glucose range compared with PID (74.4 vs. 63.7%, P = 0.021) (Table 1). Similarly, percent time in the tight target range (80–140 mg/dL) was statistically greater for MPC compared with PID (49.8 vs. 36.5%, P = 0.009) (Table 1). This improvement is also exhibited in the statistically significant improvements in high BG index (HBGI) (30) for the entire study period (4.4 vs. 7.51, P = 0.011) and the decreases in the percent time >180 mg/dL (21 vs. 33.3%, P = 0.020) (Table 1). The mean glucose values for each subject were statistically lower for MPC in comparison with PID (138 vs. 160 mg/dL, P = 0.012) (Table 1), which can also be seen in Fig. 3A, representing the mean and % time in the safe glucose range for each individual subject. Overnight control was excellent with both controllers, showing similar times in the safe glucose range (89.1 and 83.7%) (Table 1).
Both closed-loop controllers minimized hypoglycemia with regard to both the number of hypoglycemic events requiring treatment and the duration of the events (% time <70 mg/dL) (Table 1), with no significant differences between groups. The majority of hypoglycemia treatments occurred in a small cohort of subjects for both arms of the study (Supplementary Fig. 2).
Analysis of 5-h postprandial glucose levels for the dinner meal showed no differences between the controllers (Supplementary Table 3). Although time in range 70–180 mg/dL was also similar after the 50-g breakfast (Supplementary Table 3), time in range 80–140 mg/dL was statistically improved for MPC versus PID (38.1 vs. 20.9%, P = 0.008) (Supplementary Table 3).
After the unannounced 65-g lunch, mean glucose during the 5-h postprandial period was 181 mg/dL for MPC and 220 mg/dL for PID (P = 0.019) (Table 1 and Fig. 3D), with similar results for change in glucose from premeal baseline (146 and 142 mg/dL, respectively) and percent time in the safe glucose range. As expected, some subjects, particularly those who chose to consume meals with a high fat content (30–60 g), experienced greater degrees of hyperglycemia after the unannounced meal, with one subject requesting to end the session early under PID control per protocol for persistent hyperglycemia requiring a correction bolus.
There were no differences in the overall insulin delivery between MPC and PID (Fig. 1C, Supplementary Table 4). Both control algorithms also delivered similar mean amounts of insulin in the 5-h period after the unannounced meal. Of note, the PID controller suspended insulin delivery more frequently than MPC (Supplementary Table 4), although the total insulin delivery was statistically similar for both MPC and PID controllers.
Individual traces for all 27.5-h CLC sessions from the study are provided in Supplementary Data.
Adverse Events
There were no unanticipated adverse events during the study. Anticipated adverse events included hyper- and hypoglycemia. Hyperglycemia in particular was anticipated owing to the unannounced high-carbohydrate meal. One subject had prolonged hyperglycemia after the unannounced meal under PID control. Although there was no significant ketone formation (<0.6 mmol/L), the subject’s glucose levels rose >250 mg/dL and remained persistently elevated. Since the subject was not comfortable continuing the trial, the session was terminated early and a manual insulin bolus was administered, as per protocol.
The HMS functioned well for predicting glucose levels <65 mg/dL and advised treatment with 16 g carbohydrates, if necessary. No subject experienced BG <50 mg/dL by fingerstick BG during the study under CLC without a prior warning from the HMS and subsequent treatment with carbohydrates. These interventions rapidly restored euglycemia. Eight subjects experienced four or more hypoglycemia treatments during a closed-loop session, as seen in Supplementary Fig. 2. Of these, 4 subjects experienced a similar number of alarms for both sessions. Although not statistically significant, MPC sessions exhibited a greater number of hypoglycemia alarms and treatments for the remaining four sessions than corresponding PID sessions.
Conclusions
In contrast to previous assertions supporting the superior performance of any one type of algorithm across different trials (31,32), this first randomized study expressly designed to directly compare the performance of MPC and PID showed that both controllers performed very well overall, even after a 65-g unannounced meal was accounted for, and did so with low rates of hypoglycemia. MPC showed a significantly greater improvement in glucose control over PID, with a statistically greater percent time in the safe glucose range throughout the entire trial period. Both controllers also performed well in the presence of an unannounced meal, with MPC exhibiting a statistically lower mean glucose value during the 5-h postprandial period in comparison with PID.
One common argument against claiming the superiority of any single control algorithm is that the algorithms are designed to achieve different objectives, such as minimizing hyperglycemia (33) or hypoglycemia (5). Other studies focused on postprandial glucose control (11). All of these factors affect how an AP control algorithm performs, creating difficulty in formulating an objective comparison of algorithms.
In this study, we focused on realistic challenges an AP may face with more widespread use. These included overnight control after a 65-g dinner, response to a 50-g breakfast, and response to an unannounced 65-g lunch. Boluses were given at mealtime for the dinner and breakfast meal. As shown in our previous studies, the AP system had responded well to unannounced meals of up to 50 g carbohydrates (6,7). This study included an unannounced meal of 65 g carbohydrates for lunch the second day because missed meal boluses are likely to occur for practical use of AP systems. Moreover, while some studies incorporated a bedtime snack or elevated control targets to prevent nocturnal hypoglycemia (34), both the MPC and PID controllers in this study were challenged with no changes to the controller settings overnight and the subjects' normal meal plan for bedtime.
Both controllers performed well overall and performed similarly to other studies of SAP use that did not specifically include unannounced meals (35). The response to the unannounced 65-g meal, the most difficult challenge in this study, showed overall good results, with most subjects returning to the target range by the end of the 5-h window (mean time to <180 mg/dL 157 min [MPC] and 188 min [PID], P = 0.313). Some subjects showed prolonged periods of hyperglycemia, most likely related to their consumption of meals with high fat content. This delayed hyperglycemia, seen after consumption of a high-fat meal, may improve with the introduction of automated boluses using biphasic or multiphasic patterns, as previously suggested (36). The AP system used in this study did not support the use of extended boluses, explaining the difficulties some subjects faced after consuming high–fat content meals. In addition, our results show that at 5 h after the unannounced meal, both forms of CLC delivered slightly less insulin than would have been delivered had the meal of the same carbohydrate content been delivered with announcement, as was the case for dinner, although this difference was not significant. The performance of both algorithms may be improved if the controller could better differentiate corrections for postprandial glucose elevation in comparison with elevated glucose levels not directly related to meals. In addition, a subject requested to stop a session early after the unannounced meal under PID control due to elevated glucose levels. This is a very important finding, as it should be expected that AP systems will be faced with similar challenges to the 65-g unannounced meal when CLC use is adopted by a larger population. When accounting for the 65-g unannounced meal and two large announced meals under CLC, the very high percent of time in the target glucose range for both controllers overall supports previous assertions that single hormone therapy is suitable for AP, reducing the number of interventions necessary under SAP (37).
The study was designed to compare the performance of the PID and MPC controllers under conditions that were as equitable as possible. As we have previously demonstrated, the basic MPC strategy that was used in this study can be extended with additional features, such as zone-based approach to replace the fixed set point, added velocity penalties, and asymmetric weighting of glucose excursions above or below the target (27,38). Similar versatility can be achieved for PID control using zones and switching between controller settings depending on glucose above or below the target. We chose not to use these features in order to facilitate an equitable comparison of the controllers.
We acknowledge there are many other limitations to this study. First, even with a session duration of 27.5 h closed-loop time, it is still not possible to definitively determine how the algorithms will function for prolonged time periods in the home setting. In addition, frequent meal boluses will constrain the controller from giving corrections. As we only had three meals in the study, we did not test the controller under the condition of short meal intervals. Third, although we made our best efforts to design the controllers under as equitable settings as possible, we acknowledge that there are many algorithms currently being used in different studies, and we could not compare all of them. There are also other varieties of everyday challenges that have not been tested in this study, such as exercise. Finally, we recognize that the controller is only a part of the overall AP system. Many other components used in the system, or the activities of the subject (such as performing regular exercise), may play an equal, or possibly more important, role in achieving glycemic goals. For example, two subjects (subjects nos. 15-032 and 15-036) in the study experienced undesirable glycemic variability during both CLC sessions, requiring 6 and 11 and 9 and 9 treatments, respectively, for MPC and PID sessions. This appeared to be a direct consequence of the same, or worse, variability that the subjects experienced during open-loop care due to suboptimal basal insulin delivery settings—most likely basal rates that were set too high. These few subjects experienced the highest rate of hypoglycemia treatments, increasing the overall mean number of hypoglycemia treatments under CLC, although most subjects only needed zero or one treatment (Supplementary Fig. 2). While the mean number of hypoglycemic alerts overall averaged at two per session, four PID sessions and eight MPC sessions exhibited more than two treatments per day, a threshold for number of hypoglycemia treatments that may be too high for ambulatory clinical practice. Zero alerts per day and no need for supplemental carbohydrate to treat hypoglycemia are what we aim for; however, this may be an unreasonable expectation at this development stage without increase in mean glucose and greater time spent in the hyperglycemic range. Instead, we suggest a threshold of two treatments per day as an acceptable compromise. Twenty percent of the PID clinical sessions and 40% of the MPC clinical sessions experienced more treatments than this threshold. Detuning of the aggressiveness of the controllers, as was done in previous trials; elevation of the set point; or adjustment of other constraints may be necessary to limit hypoglycemia in these subjects while still addressing the concern of overall glucose control performance (39). This is an important issue that can also be addressed with optimization of insulin delivery settings prior to initiating CLC, possibly by using run-to-run adaptation strategies with algorithmic or clinician-directed adjustment (1,40).
We conclude that the MPC controller matched or outperformed the PID controller on all clinical metrics of glucose control in this study, although both controllers provided safe and effective glucose management and appear well suited for future AP applications. Future studies should continue to report on results under nonideal conditions so that the readiness of CLC for widespread use can be ascertained.
Clinical trial reg. nos. NCT02438670 and NCT01987206, clinicaltrials.gov.
J.E.P., J.B.L., and E.D. are co–first authors and contributed equally to this study.
Article Information
Acknowledgments. The authors acknowledge Dr. David Kerr, Dr. Kristin Castorino, Dr. Alexander Morf, Tyler Jean, and Katie Tovar (William Sansum Diabetes Center), as well as all the support staff at the William Sansum Diabetes Center, for assistance with this study. The authors further acknowledge Dr. Isuru Dasanayake (Intel Corporation) for his assistance. Finally, the authors thank and acknowledge product support from Animas Corporation; Dexcom, Inc.; and LifeScan, Inc.
Funding. This work was supported by the JDRF (grant 22-2011-237) and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grants DP3-DK-094331 and R01-DK-085628).
Duality of Interest. H.C.Z. is currently employed by the Insulet Corporation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.E.P., J.B.L., and E.D. helped design the study protocol, contributed to the technology design, and ensured the regulatory approval of the CLC system; provided primary technical and clinical support on site during all sessions; analyzed data; and authored the manuscript. D.E.S. contributed to the technology design of the CLC system and edited and revised the manuscript. P.K.B. and W.C.B. conducted the clinical trials, researched data, and reviewed and edited the manuscript. R.G. helped construct the controller infrastructure, contributed to the discussion, and reviewed and edited the manuscript. L.H. helped conduct the clinical trial, contributed to the discussion, and reviewed and edited the manuscript. H.C.Z. designed the study protocol, contributed to the technology design, contributed to the discussion, and reviewed and edited the manuscript. F.J.D. edited and reviewed the manuscript and was the principal investigator of the project. F.J.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.